- Research
- Open access
- Published:
Clinical characteristics and outcomes of patients with Herpes Simplex Encephalitis in Vietnam: a retrospective study
BMC Infectious Diseases volume 24, Article number: 556 (2024)
Abstract
Background
Herpes simplex encephalitis (HSE) is an important central nervous infection with severe neurological sequelae. The aim of this study was to describe clinical characteristic and outcomes of patients with HSE in Vietnam.
Methods
This was a retrospective study of 66 patients with herpes simplex encephalitis who admitted to the National Hospital for Tropical Diseases, Hanoi, Vietnam from 2018 to 2021. The detection of herpes simplex virus (HSV) in cerebrospinal fluid was made by the real-time PCR assay. We reported the clinical manifestation on admission and evaluated clinical outcomes at the hospital discharge by modified Rankin Scale (mRS). Multivariate logistic regression analysis was used to analyze the independent risk factors of severe outcomes.
Results
Of the 66 patients with laboratory confirmed HSE, the median age was 53 years (IQR 38–60) and 44 patients (69.7%) were male. The most common manifestations included fever (100%), followed by the consciousness disorder (95.5%). Other neurological manifestation were seizures (36.4%), memory disorders (31.8%), language disorders (19.7%) and behavioral disorders (13.6%). Conventional magnetic resonance imaging (MRI) showed 93.8% patients with temporal lobe lesions, followed by abnormalities in insula (50%), frontal lobe (34.4%) and 48.4% of patients had bilateral lesions. At discharge, 19 patients (28.8%) completely recovered, 15 patients (22.7%) had mild sequelae, 28 patients (42.4%) had moderate to severe sequelae. Severe neurological sequelae were memory disorders (55.8%), movement disorders (53.5%), language disorders (30.2%). Multivariate logistic regression analysis showed that Glasgow score decrement at admission, seizures, and time duration from onset of symptoms to the start of Acyclovir treatment > 4 days were independent factors associated with severe outcomes in HSE patients.
Conclusion
Glasgow score decrement, seizures and delay treatment with Acyclovir were associated with the poor outcome of patients with HSE.
Introduction
Herpes simplex virus (HSV) encephalitis is a life-threatening central nervous system infection associated with poor outcomes [1]. It occurs sporadically with a frequency of 2–4 cases per 1,000,000 population per year in high-income countries [2]. Over 90% of HSE cases are caused by HSV-1, with the remainder attributed to HSV-2 [3].
Prior to the availability of effective antiviral treatment, the mortality rate of encephalitis caused by HSV was about 70% [4]. In the 1980s, the introduction of intravenous Acyclovir significantly improved the prognosis of HSE, reducing the mortality rate to only 6-11% [3]. In the 1990s, the use of polymerase chain reaction (PCR) for HSV detection in the cerebrospinal fluid further enhanced early prognosis and treatment [5]. However, the rate of neurological sequelae remains high, happening in 44–62% of the surviving patients [6] with the delay in antiviral initiation as the most common reason [7,8,9].
In the context of Vietnam, HSE constitutes 7% of pediatric cases with acute encephalitis [10] and around 3% of suspected central nervous system (CNS) infections in adults [11]. However, research on this severe condition, both domestically and internationally, is restricted, due to the low incidence of this disease and the prevalence of small-scale studies primarily focusing on clinical symptoms. This study aims to address this gap by providing a comprehensive analysis of the clinical characteristics and prognostic factors linked to poor outcomes in HSE patients using a substantial cohort of cases from 2018 to 2021 at the National Hospital for Tropical Diseases (NHTD), a tertiary hospital specialized in treating infectious diseases.
Materials & methods
Study design
This retrospective study analyses medical records of all patients who were diagnosed with HSE at the National Hospital for Tropical Diseases (NHTD), which is a referral hospital for treatment of infectious diseases in the North of Vietnam, from July 1, 2018, to June 30, 2021. The inclusion criteria of patients included: (1) over 18 years of age, (2) suspected encephalitis according to the guidelines of the International Encephalitis Association (2013) [12] and (3) had positive HSV DNA in cerebrospinal fluid tests using Real-time PCR assay (Bio-Rad Iq5 Multicolor Real Time PCR Detection System and Chromo4 real-time PCR detection system). Criteria for suspected encephalitis consisted of major criterion of altered mental status (defined as decreased or altered level of consciousness, coma, or personality changes) lasting ≥ 24 h with no other identifiable cause and at least of 2 minor criteria of fever, generalized or partial convulsions, new onset of focal neurologic findings, CSF with lymphocytosis (≥ 5 white blood cells/µl) and brain parenchymal abnormalities on neuroimaging or EEG suggestive of encephalitis [12]. Patients with pre-existing brain injury, history of mental disorders, inability to communicate normally (mute or deaf), or concurrent bacterial meningitis were excluded from the study. The protocol of this study was approved by the Ethics Committee and review board of the National Hospital for Tropical Diseases (IORG0006480) with approval number 9 A/HDDD-NĐTƯ dated 22, June 2021. As the study was conducted retrospectively, the informed consent waiver was accepted by the Ethics Committee and review board of the National Hospital for Tropical Diseases.
Data collection and outcome assessment
We collected information of (1) demographics, (2) clinical symptoms and signs, (3) results of laboratory testing and cerebrospinal fluid analysis at admission and during the hospitalization, (4) reports of brain MRI and CT, and (5) treatment (Acyclovir, mechanical ventilator).
The patient’s outcomes at the time of discharge were evaluated using the modified Rankin scale (mRS) [13]. The scores of mRS were classified as follow: 0 (No sequelae), 1 (No significant sequelae despite having symptoms, capable of performing all usual tasks and activities), 2 (Slight sequelae, unable carry out all previous activities, but able to look after own affairs without assistance), 3 (Moderate sequelae, requiring some help but able to walk without assistance), 4 (Moderately severe sequelae, unable to wall or attend to own bodily needs without assistance), 5 (Severe sequelae, being bedridden, incontinent and requiring constant nursing care or attention) and 6 (Dead). The outcomes of the patients were categorized as mild if the mRs score was < 3 or as severe if the mRS score was ≥ 3.
Statistical analysis
SPSS software version 20.0 was used for statistical analysis. Descriptive analysis was performed to explore the demography, clinical manifestations, and laboratory results. As the data were not normally distributed, non-parametric tests were used for statistical analysis, specifically the Mann-Whitney U test to compare the median of the quantitative variables and χ2 test to compare the proportions of the qualitative variables. While a preliminary analysis was published on a subset of participants in Vietnam Medical Journal [14] this work was performed on the completed sample using the univariate and multivariate logistic regression analysis to explore the independent factors associated with disease severity. The statistically significant cutoff was p < 0.05.
Results
Patient characteristics
66 eligible patients were included in the study, with a median age of 53 (IQR, 38–60 years), of whom 46 patients (69.7%) were male. There were 30.3% of patients who had at least one comorbidity, in which the most common comorbidity was cardiovascular diseases (18.2%), followed by diabetes (9.1%). Most of the patients (90.9%) were transferred from primary and secondary-level hospitals to the NHTD. The median time from the onset of symptoms to hospital admission was approximately 5.5 days (IQR 3–7 days) while the median time from the onset of symptoms to administration of Acyclovir was 6.0 days. The clinical manifestation and laboratory results were showed in the Table 1. Fever was presented in all patients while meningeal signs were apparent in 57 patients (86.4%). 63 patients (95.5%) were admitted with disturbed consciousness, in which 24 patients (36.4%) had seizures. The median of the GCS is 13, which has been rounded up to preserve its clinical significance. 25 patients (37.9%) required admission to the intensive care unit, in which the majority of patients required mechanical ventilation and developed ventilator associated pneumonia.
64 patients (97%) had brain MRI results. The median duration from the onset of symptoms to the MRI examination was 6 (IQR, 4–9) days with the common lesions being observed in the temporal lobe (93.8%), insular lobe (50%), and frontal lobe (34.4%).
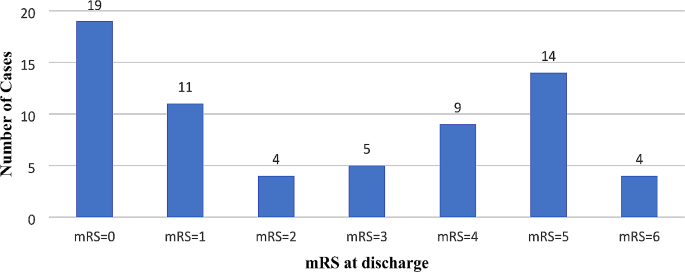
Figure 1. illustrates patient outcomes at discharge: 28.8% achieved full recovery (mRS = 0), 22.7% had mild sequelae (0 < mRS ≤ 2), 42.4% experienced moderate to severe sequelae (3 ≤ mRS ≤ 5), and 4 deaths. Of the 43 patients with sequelae, common symptoms included memory disorders (55.8%), movement disorders (53.5%), and language disorders (30.2%).
Prognostic factors for severe outcome
The differences in the frequency of symptoms and laboratory results on admission according to mRS were showed in the Tables 2 and 3. The patients with mRS score ≥ 3 had higher rate of seizures (56.2% vs. 17.6%), lower GCS score (12 points vs. 13 points), higher rate of hemiplegia (15.6% vs. 0%), and more frequency of multiple lobar involvements in MRI (46.7% vs. 14.7%).
Multivariate logistic regression analysis showed that lower GCS at admission (aOR: 0.25–0.76 per 1 point increment), seizure (aOR 1.65–37.14) and time from onset to the start of Acyclovir treatment > 4 days (aOR 1.48–44.31) were independent factors related to severe prognosis in patients with HSE, as shown in Table 4.
Discussion
A study between 2014 and 2017 in Vietnam reveals that the main pathogens responsible for CNS infections among 137 patients were Streptococccus suis (12%) and Neisseria meningitidis (7%), followed by HSV (3%) [15]. Similarly, another study in Southern Vietnam over the duration of 12 years (1996–2008) found that HSV ranked 2nd among viral etiologies for CNS infection (6.5%) after Japanese encephalitis virus (12%) [16]. Our study is the first to focus on HSE in the Northern Vietnamese regions with a significant number of patients. Within the scope of our research, we found that the in-hospital mortality was at 6.1%, which was similar to the those reported by studies in both France (5.5%) [17] and India (6.8%) [18]. Additionally, we also identified an association between poor outcomes in HSE patients and neurological symptoms as well as delays in antiviral initiations, serving as potential prognosis factors.
Among the symptoms, consciousness status at the time of admission determined by the GCS score is strongly associated with poor outcomes in HSE patients. According to our analysis, the lower the GCS, the higher the risk of being discharged from hospital in severe condition. The probability of hospital discharge with severe conditions decreases by 0.44 times when GCS increases by 1 point. This result is in accordance with that of the study by Kamei et al., in which the same probability decreases by 1.45 times per 1 increment of GCS score before treatment [19]. Although we did not focus on the threshold of GCS as a prognostic factor, previous reports have discussed this. A multinational study by Erdem et al. in 2015 with 438 patients showed that those with a GCS score of less than 5 experienced poor outcomes more frequently [20]. Similarly, Whitley also assessed the prognostic capability of GCS score across all age ranges and confirmed that a GCS score of less than 6 can predict a poor outcome in HSE patients, irrespective of the medicine administered or the age of the patients [21].
Along with worsening consciousness, seizure is another clinical manifestation of HSE patients, with an incidence rate ranging from 35 to 65% [22]. Our analysis indicates that seizure on admission is an independent prognosis of severity. In particular, patients with seizures are 7.84 times more likely in a severe condition than those without. Therefore, the presence of seizures should also be closely monitored in HSE patients.
In treating HSE patients, the administration of Acyclovir has been reported to be of paramount importance, especially during the early stage of the disease [8, 20, 23, 24]. Correspondingly, our analysis found that patients who received Acyclovir 4 days after the onset of symptoms are 8.1 times more likely to be in severe conditions, indicating that it is more beneficial for patients to be administered Acyclovir early. However, the benefits of the empirical use of Acyclovir in patients likely diagnosed with HSE without any confirmation from molecular tests such as HSV CFS PCR tests have not been clinically proven. In the study by Benson and Swadron, 17 out of 24 patients (71%) with suspected HSE was not administered with Acyclovir in the emergency department but rather in in-patient settings, though the morbidity and mortality of the patients were not examined due to the short study length [25]. In addition, despite having high sensitivity and specificity [21], standard HSV CFS PCR test requires long time for confirmation and also poses possibility of false positives [26, 27], leading to our proposal that the decision to treat HSE early should be based on clinical evaluation and brain imaging rather than deferring treatments until the PCR result is available.
Due to the retrospective nature of our study, we were restricted to evaluate the outcomes of the patients at the time of hospital discharge, and we could not follow up on the sequelae caused by HSE for a longer term. It is also probable that there are some restrictions in comparing the clinical characteristics because the results did not correspond to the disease progression of the patients included in the study as well as the referral system from tertiary healthcare centers might cause delays in recording such characteristics.
Conclusion
HSE was associated with poor outcome and high case-fatality at hospital discharge in Vietnamese patients. This study highlights the importance of early diagnosis and empirical treatment in the lower level of healthcare system in Vietnam to improve the clinical outcome.
Data availability
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- CRP:
-
C reactive protein
- HSE:
-
Herpes simplex encephalitis
- HSV:
-
Herpes simplex virus
- GCS:
-
Glasgow Coma Score
- MRI:
-
Magnetic resonance imaging
- mRS:
-
Modified Rankin Scale
- NHTD:
-
National Hospital for Tropical Diseases
- PCR:
-
Polymerase Chain Reaction
- WBC:
-
White blood cell
References
Rui-Yun Zhang. Research on early diagnosis and impact prognostic factors of herpes simplex encephalitis. Int J Clin Exp Med. 2016;:9(2):4695–8.
Solomon T, Hart IJ, Beeching NJ. Viral encephalitis: a clinician’s guide. Pract Neurol. 2007;7:288–305.
Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret’s. Herpes J IHMF. 2004;11(Suppl 2):A57–64.
Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch’ien LT, Alford CA. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious diseases collaborative antiviral study. N Engl J Med. 1977;297:289–94.
Aurelius E, Johansson B, Sköldenberg B, Staland A, Forsgren M. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet Lond Engl. 1991;337:189–92.
Whitley RJ, Alford CA, Hirsch MS, Schooley RT, Luby JP, Aoki FY, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–9.
Dagsdóttir HM, Sigurðardóttir B, Gottfreðsson M, Kristjánsson M, Löve A, Baldvinsdóttir GE, et al. Herpes simplex encephalitis in Iceland 1987–2011. SpringerPlus. 2014;3:524.
Sili U, Kaya A, Mert A. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–8.
Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, et al. Outcome of and prognostic factors for Herpes Simplex Encephalitis in Adult patients: results of a Multicenter Study. Clin Infect Dis. 2002;35:254–60.
Bich HT, Van Lam N, Hoang Thi Hoa. Complications of Encephalitis and Health Care Needs at Vietnam National Children’s hospital (2018–2019). J Pediatr Res Pract. 2020;4:41–8.
Taylor WR, Nguyen K, Nguyen D, Nguyen H, Horby P, Nguyen HL, et al. The Spectrum of Central Nervous System infections in an Adult Referral Hospital in Hanoi, Vietnam. PLoS ONE. 2012;7:e42099.
Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, Diagnostic algorithms, and priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin Infect Dis. 2013;57:1114–28.
Riera-Mestre A, Gubieras L, Martínez-Yelamos S, Cabellos C, Fernández-Viladrich P. Adult herpes simplex encephalitis: fifteen years’ experience. Enfermedades Infecc Microbiol Clínica. 2009;27:143–7.
Ta Thi Dieu Ngan, Nguyen Thi Tuyet. Evaluation the outcomes of herpes simplex encephalitis in patients treated at National Hospital for Tropical Diseases from the year 2018 to 2022. Vietnam Med J. 2023;523:20–5.
Gabor JJ, Anh CX, Sy BT, Hoan PQ, Quyen DT, The NT, et al. Aetiologies and clinical presentation of central nervous system infections in Vietnamese patients: a prospective study. Sci Rep. 2022;12:18065.
Tan LV, Thai LH, Phu NH, Nghia HDT, Chuong LV, Sinh DX, et al. Viral aetiology of central nervous system infections in adults admitted to a tertiary referral hospital in southern Vietnam over 12 years. PLoS Negl Trop Dis. 2014;8:e3127.
Jouan Y, Grammatico-Guillon L, Espitalier F, Cazals X, François P, Guillon A. Long-term outcome of severe herpes simplex encephalitis: a population-based observational study. Crit Care. 2015;19:345.
Jain P, Jain A, Kumar A, Prakash S, Khan DN, Singh KP, et al. Epidemiology and etiology of Acute Encephalitis Syndrome in North India. Jpn J Infect Dis. 2014;67:197–203.
Kamei S, Sekizawa T, Shiota H, Mizutani T, Itoyama Y, Takasu T, et al. Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry. 2005;76:1544–9.
Erdem H, Cag Y, Ozturk-Engin D, Defres S, Kaya S, Larsen L, et al. Results of a multinational study Suggest the need for Rapid Diagnosis and early antiviral treatment at the Onset of Herpetic Meningoencephalitis. Antimicrob Agents Chemother. 2015;59:3084–9.
Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–8.
Gnann JW, Whitley RJ. Herpes Simplex Encephalitis: an update. Curr Infect Dis Rep. 2017;19:13.
Stahl JP, Mailles A, Broucker TD. Group the SC and I. herpes simplex encephalitis and management of acyclovir in encephalitis patients in France. Epidemiol Infect. 2012;140:372–81.
Sheybani F, Arabikhan HR, Naderi HR. Herpes Simplex Encephalitis (HSE) and its outcome in the patients who were admitted to a Tertiary Care Hospital in Mashhad, Iran, over a 10-year period. J Clin Diagn Res JCDR. 2013;7:1626–8.
Benson PC, Swadron SP. Empiric acyclovir is infrequently initiated in the Emergency Department to patients ultimately diagnosed with encephalitis. Ann Emerg Med. 2006;47:100–5.
Weil AA, Glaser CA, Amad Z, Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis off Publ Infect Dis Soc Am. 2002;34:1154–7.
Adler AC, Kadimi S, Apaloo C, Marcu C. Herpes simplex encephalitis with two false-negative cerebrospinal fluid PCR tests and review of negative PCR results in the clinical setting. Case Rep Neurol. 2011;3:172–8.
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
T.T.D.N. designed the study and drafted the manuscript. N.T.T. collected, analyzed, and interpreted the data. D.M.N. interpreted the data and drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of research has been approved by the Ethics Committee and review board of the National Hospital for Tropical Diseases (IORG0006480), approval number 9 A/HĐĐĐ-NĐTƯ dated on June 22, 2021. The Ethics Committee and review board of the National Hospital for Tropical Disease also approved the waiver of informed consent for participation due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ngan, T.T.D., Tuyet, N.T., Hung, D.T. et al. Clinical characteristics and outcomes of patients with Herpes Simplex Encephalitis in Vietnam: a retrospective study. BMC Infect Dis 24, 556 (2024). https://doi.org/10.1186/s12879-024-09453-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09453-3