- Study Protocol
- Open access
- Published:
Evaluation of serological assays for the diagnosis of childhood tuberculosis disease: a study protocol
BMC Infectious Diseases volume 24, Article number: 481 (2024)
Abstract
Background
Tuberculosis (TB) poses a major public health challenge, particularly in children. A substantial proportion of children with TB disease remain undetected and unconfirmed. Therefore, there is an urgent need for a highly sensitive point-of-care test. This study aims to assess the performance of serological assays based on various antigen targets and antibody properties in distinguishing children (0–18 years) with TB disease (1) from healthy TB-exposed children, (2) children with non-TB lower respiratory tract infections, and (3) from children with TB infection.
Methods
The study will use biobanked plasma samples collected from three prospective multicentric diagnostic observational studies: the Childhood TB in Switzerland (CITRUS) study, the Pediatric TB Research Network in Spain (pTBred), and the Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infections in children and adolescents (ProPAED) study. Included are children diagnosed with TB disease or infection, healthy TB-exposed children, and sick children with non-TB lower respiratory tract infection. Serological multiplex assays will be performed to identify M. tuberculosis antigen-specific antibody features, including isotypes, subclasses, Fc receptor (FcR) binding, and IgG glycosylation.
Discussion
The findings from this study will help to design serological assays for diagnosing TB disease in children. Importantly, those assays could easily be developed as low-cost point-of-care tests, thereby offering a potential solution for resource-constrained settings.
ClinicalTrials.gov Identifier
NCT03044509.
Background
Diagnosing tuberculosis (TB) in children presents several challenges [1]. TB disease in children is confirmed only in about 50% of patients due to the paucibacillary nature [2, 3]. In the absence of a reliable and easily accessible diagnostic test for screening and confirming TB disease in children, diagnosis typically relies on clinical findings, TB contact history, chest radiography findings, and the results of immune-based TB tests, the Tuberculin skin test (TST) and interferon-γ release assays (IGRA) [4]. However, both immunodiagnostic tests have suboptimal performance and are not well-suited for screening for TB disease [5, 6].
Serological assays have the potential to serve as a screening tool for TB infection and disease in children, especially in resource-limited settings where advanced diagnostic methods are limited. This potential stems from their blood-based nature, thus not requiring sputum collection, and their feasibility to be used as point-of-care tests [7]. However, currently available commercial serological assays are not recommended for clinical use due to their insufficient and variable diagnostic performance, characterised by limited sensitivity, specificity, and susceptibility to cross-reactivity [8, 9]. In a recent narrative review focusing on the diagnostic performance of non-commercial serological assays for TB in children, we found that studies which measured antibodies against only one antigen generally reported relatively high specificity but only achieved limited sensitivity [10]. Higher sensitivity can be achieved when antibodies against multiple targets are measured, and results are interpreted in combination. In addition, emerging evidence suggests that certain antibody properties, such as antibody Fc receptor (FcR) binding profiles [11, 12] and antibody glycosylation patterns [13], can potentially be used to differentiate between TB infection and disease. However, most of those studies have been done in adults, and the evidence in children remains extremely limited.
Methods
Aim
The aim of this study is to evaluate the diagnostic performance of serological assays in detecting children with TB disease, and in distinguishing those subjects from (1) healthy TB-exposed children, (2) children with non-TB lower respiratory tract infection, and (3) children with TB infection.
Study setting and population
This study will utilise plasma samples obtained from three different prospective multicentric observational studies: the Childhood Tuberculosis in Switzerland (CITRUS) study (NCT03044509), the Pediatric TB Research Network in Spain (pTBred), and the Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infections in children and adolescents (ProPAED) study (ISRCTN 17,057,980) (Table 1).
CITRUS is a multicentric prospective diagnostic study done at nine centres across Switzerland (Bern, Basel, Zurich, Lausanne, Geneva, Aarau, St. Gallen, Lucerne, Bellinzona). Its primary objective is to evaluate and validate novel immunodiagnostic assays for childhood TB [14, 15]. The study includes children under the age of 18 years, with or without a history of Bacillus Calmette-Guérin (BCG) vaccination, who are undergoing evaluation for TB disease, infection, and exposure. Children who have received any anti-mycobacterial treatment for five days or more before inclusion or who have been previously treated for TB disease or infection are excluded. Recruitment for the CITRUS study began in May 2017 and is currently ongoing.
PTBred is a multidisciplinary collaborative network established in 2014 in Spain, recruiting children < 18 years with TB. Since 2017, different types of samples have been stored in the Biobank of the Gregorio Marañon Hospital or in the individual collection registered as C.0006631 in the National Biobank Collections Registry. For this study, a common protocol for sample processing was implemented in October 2019, including children with children with TB disease, infection, and exposure irrespective of their BCG-vaccination status. The pTBred and CITRUS study follow the same inclusion and exclusion criteria [16].
The ProPAED study collected samples from children and adolescents presenting with fever and cough at two emergency departments in Switzerland (Basel and Aarau), from January 2009 to February 2010. For the ProPAED study, children with severe immunocompromise or known HIV infection, those undergoing immunosuppressive treatment, children with M. tuberculosis infection, neutropenia, cystic fibrosis, viral laryngotracheitis, hospital stay within the preceding 14 days, or other severe infections (e.g., osteomyelitis, endocarditis, or deep tissue abscesses) were excluded [17].
Case definitions
In this study, we will use the published criteria of compound TB case definitions proposed by Graham et al. [18]. Briefly, confirmed TB disease is defined as the presence of bacteriologically confirmed TB disease through culture or nucleic acid amplification tests (NAAT). Unconfirmed TB disease is defined as the absence of bacteriological confirmation in the presence of at least two of the following criteria: symptoms or signs suggestive of TB disease, chest radiograph consistent with TB disease, close TB exposure or immunologic evidence of M. tuberculosis infection, positive response to TB treatment. TB infection is defined as the presence of immunologic evidence of M. tuberculosis infection, including a positive TST of ≥ 5 mm (in accordance with the Swiss and Spanish guidelines [19, 20]) or a positive IGRA without meeting the criteria for confirmed or unconfirmed TB disease. Healthy TB-exposed children are defined as asymptomatic individuals with negative results on IGRA or TST test (single or repeat testing according to age, time since exposure as defined by national guidelines), making them unlikely to have TB. Children with non-TB lower respiratory tract infection will be the sick control group and are defined as presenting with fever (core body temperature ≥ 38.0° C) and at least one symptom (cough, sputum production, pleuritic pain, poor feeding) and at least one sign (tachypnea, dyspnoea, wheezing, late inspiratory crackles, bronchial breathing, pleural rub) lasting for fewer than 14 days.
Age stratification
The study will analyse antibody concentrations and properties in children stratified into distinct age groups: 0 to < 2, 2 to < 5, 5 to < 10, and ≥ 10 years, as proposed by Cuevas et al. [21]. This stratification is crucial due to the differences and dynamics of the nature of TB disease across age. In the youngest age range (infants and children < 2 years old), disseminated diseases and heightened susceptibility to progression from TB infection to TB disease is well-documented [22]. The risks for progression from infection to disease, as well as the subsequent mortality risk following development of disease, consistently declines during childhood, reaching its lowest point between 5 and 10 years of age [23]. Transitioning into adolescence and the onset of puberty, typically beyond the age of 10 years, the phenotype of TB disease becomes more adult-like. Pulmonary TB becomes more prevalent during this phase, contributing to an upsurge in TB-related mortality rates [24, 25].
Selected antigen targets and antibody properties for serological assay
Some previous studies in children have demonstrated improved specificities achieved by combining both protein and glycolipid antigens within serological assays [26,27,28,29]. Furthermore, several studies have illuminated the potential for heightened sensitivity through the combined analysis of multiple antigen targets, effectively overcoming the interindividual heterogeneity of the human humoral immune response to M. tuberculosis [26,27,28,29,30,31,32,33].
We will analyse antibodies concentrations and properties against single protein antigens, single glycolipid antigens [12, 34,35,36,37,38,39,40], as well as multiple antigens in combination (Table 2). The types of antigens include cell wall fractions, whole cell lysates, and total lipids of M. tuberculosis. The selection of protein antigens is based on results from large protein microarray studies in adults [41,42,43,44,45,46], one large multiplex bead-based study in children [31], and published and unpublished data from an adult study performed in the U.K (MIMIC study; personal communication M. Tebruegge) [47]. In order to enhance specificity, the overlap of the antigen targets for M. tuberculosis with Bacillus Calmette-Guérin (BCG) and other non-tuberculous mycobacteria will be reduced.
Together with targeted M. tuberculosis antigens, this study will evaluate the following distinct properties of the antibodies: isotypes and their subclasses, FcR binding profiles, and antibody glycosylation patterns (refer to Fig. 1). The rational for this is to obtain further information about the immune response to the antigen. TB disease results from a combination of the mycobacteria infecting and the resulting pathologic immune response. Therefore, antibody concentrations may only reflect on exposure, timepoint, and burden of mycobacteria, whereas additional properties such as FcR may reflect on the fact if the immune response producing tissue damage and pathology or not. This is shown in studies in children with TB disease that have demonstrated the potential enhancement of serological assay sensitivity through the integration of diverse antibody isotypes [48,49,50]. Recent advancements in adult research have indicated that an evaluation of certain antibody properties, such as FcRs binding profiles and glycosylation patterns, could potentially enable the differentiation between TB disease and infection [12, 13].
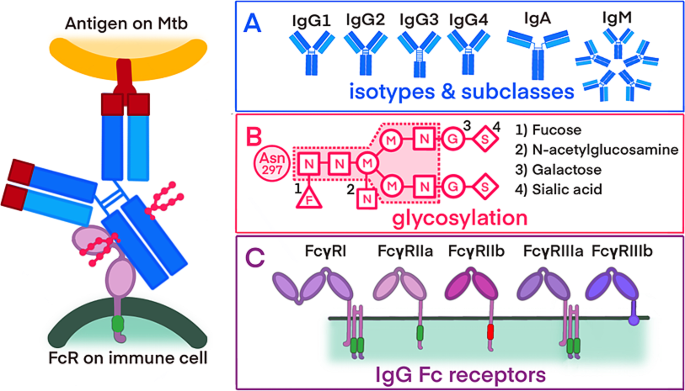
Overview of the antibody properties
Interaction between the surface of M. tuberculosis, binding of the antibody and the recognition of the antibody by an immune cell. Sections A, B, and C detail the different antibody properties: A) antibody isotypes and IgG subclasses B) glycosylation patterns of antibodies, including a core glycan and potential additional sugar residues (1–4) C) activating and inhibiting FcRs with varying affinities for antibody binding
Abbreviations: Mtb -Mycobacterium tuberculosis; FcR -fragmented crystallizable region (Fc) receptor; IgM - immunoglobulin M; IgD - immunoglobulin D, IgG1 − 4 - immunoglobulin G1 − 4; IgA - immunoglobulin A, N - N-acetylglucosamine; M - mannose; G - galactose; S - sialic acid; F - fucose
As a quality control and potential normalisation variable, we will measure the total antibody concentration of each isotype and the total antibody concentration binding to distinct FcRs.
Sample preparation
Upon plasma sample collection, preservation is ensured through storage in a − 80 °C freezer until the initiation of laboratory assays. Customised multiplex antigen-coupled beads will be produced to evaluate antigen-specific antibodies concentrations and properties in plasma samples. The protein antigens will be coupled to carboxylated beads through covalent NHS-ester linkages, using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and Sulfo-NHS (Thermo Scientific), following the manufacturer’s recommendations [51, 52]. Glycan antigen LAM, single lipid antigens (e.g., TDM and TMM), and multiple lipid antigen from Mycobacterium tuberculosis total lipids will be modified using 4-(4,6-dimethoxy [1, 3, 5] triazin-2-yl)-4-methyl-morpholinium (DMTMM) dissolved in ethanol and conjugated beads following the COOH-DMTMM method [53].
The antigen-specific antibodies concentrations and properties will be measured using different PE-labelled detection antibodies as follows: for the isotypes and subclasses, PE-coupled detection antibodies (anti-IgG, anti-IgA, anti-IgM, anti-IgG1, anti-IgG2, anti-IgG3 and anti-IgG4) at a concentration of 1 µg/mL; [52] for the FcR binding profiles, FcRs (FcγRIIIa/CD16a, FcγRIIIb/CD16b, FcγRIIa/CD32a H167, FcγRIIb/CD32b, FcγRI/CD64 from R&D Systems) will be labelled with PE and added to the samples at a concentration of 1 µg/mL; and for the glycosylation profiles, PE-labelled lectins (SNA for sialic acid, ECL for galactose, LCA for fucose and PHA-E for N-acetylglucosamine) will be used at a concentration of 20 µg/mL. After 2 h of incubation at room temperature, the beads will be washed with PBS-0.05% Tween20, and PE signal will be measured using xMAP technology. (refer to Fig. 2)
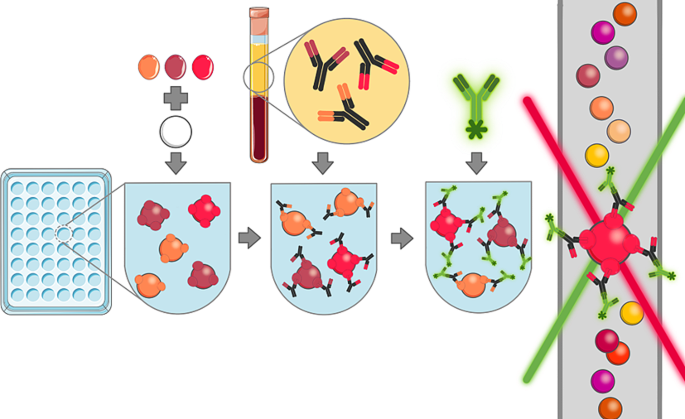
Multiplex bead-based serological assay
For the multiplex bead-base serological assay (1) specific antigens are coupled to beads, (2) plasma samples are incubated with the antigen-coupled beads, allowing specific antibodies to bind to corresponding antigens, (3) fluorescently labelled detection antibodies are added, binding to antigen-specific antibodies or their properties, (4) fluorescence is measured by using a coloured laser, and concentrations are then calculated based on a standard curve
Data management
All data will be securely entered and shared through password-protected and encrypted systems to uphold the confidentiality of health-related personal information. Adhering to Swiss legal requirements for data protection (Ordinance HRO Art. 5), our procedures for storing biological samples and handling health data are meticulously governed. Coding mechanisms and personalised logins are implemented to grant exclusive access to the study database and source documents for authorised personnel, thereby preventing third-party disclosure. Unique identification numbers are assigned to the biological samples and health-related personal data.
Data analysis
Descriptive statistics, including mean, median, standard deviation, and interquartile range, will be used to summarise antibody concentrations stratified by diagnostic group (TB disease, TB infection, healthy TB-exposed controls, and non-TB lower respiratory tract infections) and age groups (< 2 years, 2 to < 5 years, 5 to < 10 years, and ≥ 10 years). Antigen-specific antibody concentrations will be analysed in relation to the total (nonspecific) antibody concentrations. Comparisons between groups will be made using t-tests or Mann-Whitney U tests if normality assumptions are not met. Children with TB disease and infection will be compared with the following groups: all other remaining children combined, healthy TB-exposed children, and children with non-TB lower respiratory tract infections.
To assess the performance of each individual antigen specific antibody feature as a diagnostic assay, sensitivity and specificity will be calculated based on cut-off values determined by the highest Youden’s index. Receiver operating characteristic (ROC) analysis will be performed, and area under the curve (AUC) will be calculated (confidence interval will be determined using the DeLong method).
In subsequent analyses, we aim to evaluate the combined interpretation of antigen-specific antibodies concentrations and properties using different strategies:
Strategy one involves defining cut-off values based on a specificity of ≥ 98%, in accordance with the minimal WHO’s target product profile(TPP) requirement for a biomarker-based detection test. We will calculate the corresponding sensitivity. Similarly, we will determine cut-off values based on a sensitivity of ≥ 66% and calculate the corresponding specificity. To assess the combined interpretation of multiple antigen targets, the test for a specific antibody or antibody property will be scored positive if at least one antibody level against a specific antigen exceeds the cut-off value in an individual’s plasma sample, and negative if all antibody levels against all antigens in a plasma sample are below the cut-off values.
Another strategy for the combined interpretation of multiple antibody concentrations and properties will involve feature selection using the least absolute shrinkage and selection operator (LASSO). This approach will help identifying the most informative features that could be used in diagnostic assays. To validate the predictive power of the selected features (k features), we will train and evaluate an additional model using only those k features. In a further step, we will include the selection of antibody concentrations and properties in the training of the model. By performing feature selection using LASSO, we aim to maximize prediction performance using all features and select the k most informative features after the training stage. This procedure is based on the concept that selecting the most informative features from a well-performing prediction model will also yield a well-performing prediction model when one only has access to the selected subset of features. Recent advances in machine learning research will enable us to incorporate feature subset selection directly into the training step of a model [54, 55]. Therefore, we optimise not only the prediction performance but also the subset selection of k features during training. The choice of subset size, k, should be based on external constraints. The diverse sensitivities and specificities observed in paediatric TB serological tests make a precise sample size determination challenging. To estimate the sample size for our experiments, we used data generated from a cohort of adults with latent infection (n = 20) and active pulmonary disease (n = 22) from South Africa [56]. For the analysis of 75 antibody features, linear regression was conducted to assess the association between diagnosis and antibody feature, while controlling for age and gender. For the thirteen features exceeding a false discovery rate threshold of 10%, the partial correlation coefficient of 0.50 or higher was observed between diagnosis and antibody feature. Using this estimate as the effect size of biologically active antibody features, 68 individuals in an independent cohort (34 LTB, 34 ATB) would provide a statistical power of 80% to observe significant differences in top antibody features between tuberculosis infection and disease at an alpha level of 0.0005. This alpha level represents the threshold for significance required by the Bonferroni-Holm correction method, set at 0.0005 to accommodate the testing of 100 antibody features.
Publication and dissemination policy
Findings of this study will be disseminated through peer-reviewed journals, scientific conferences, and other relevant platforms. Participants will receive a summary of the results. All scientific data generated from this project will be made available as soon as possible, and no later than the time of publication or the end of the funding period, whichever comes first. The data and related metadata underlying reported findings will be deposited in a public data repository. A data access committee will support third parties who wish to perform further research with the data. Data will be curated in the repository following accepted standards and a persistent identifier, a DOI, is created for each data set published. If intellectual property is developed, dissemination of data will occur after appropriate protections for intellectual property are put in place.
Discussion
The development of reliable point-of-care tests for detecting TB infection and disease in children is crucial. Serological assays offer a promising approach, as they may be used in a point-of-care test format, making them suitable for widespread implementation in diverse settings [7]. However, there are several hurdles that need to be addressed to advance the development of TB serological assays. One challenge is the incomplete understanding of the immunogenic properties of the numerous potential antigens of M. tuberculosis, including proteins and glycolipids [57]. Our study has four main strengths. First, our study will evaluate antibodies against a broad range of protein antigens [41, 45, 46, 58], as well as glycolipids that are believed to play a crucial role in the pathogenesis of M. tuberculosis [59, 60].
Second, to overcome the challenge of potential cross-reactivity of antibodies detected in a serological assay for TB with BCG- and non-tuberculous mycobacteria-antigens [25], we will include a large range of antibodies and reduced the overlap between M. tuberculosis and BCG/non-tuberculous mycobacteria-antigens selected. Third, there exists substantial interindividual heterogeneity in the antibody response to M. tuberculosis [61, 62]. Different individuals may react to different antigens, resulting in relatively low sensitivity but good specificity for each individual antigen serological assays [30, 31, 49]. To account for this heterogeneity, our analysis includes multiple antigen targets, such as cell wall fractions and total lipids, and aims at a combined interpretation of these parameters.
Finally, we will evaluate specific antibody properties, such as antibody isotypes, glycosylation patterns, and FcR binding profiles [12]. So far, IgG is the most extensively studied isotype and has shown the most promising results for use in diagnostic assays to detect TB disease in children. Other isotypes, such as IgA, have gained attention more recently, as these have a protective role in human and animal studies in preventing TB infection [63, 64]. Glycosylation of the Fc region affects the binding affinity of the antibody to the FcRs. Notably, distinct glycosylation patterns have been associated with various stages of TB disease and infection [11]. Lastly, our data analysis is stratified across distinct age groups to accommodate the dynamic nature of TB disease during various developmental stages of children.
The findings of our study will improve our understanding of the human humoral immune response to M. tuberculosis infection and disease and holds the potential to pave the way for designing antibody-based assays with high performance characteristic for use in children.
Data availability
Data supporting this study protocol is comprehensively presented within the manuscript. For additional details or inquiries regarding the dataset, kindly reach out to the Corresponding Author, Prof. Nicole Ritz, MD/PhD, nicole.ritz@unibas.ch.
Change history
24 June 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12879-024-09432-8
Abbreviations
- AUC:
-
Area under the curve
- BCG:
-
Bacillus Calmette-Guérin
- CITRUS:
-
Childhood tuberculosis in Switzerland study
- FcR:
-
Fragmented crystallizable region (Fc) receptor
- IGRA:
-
Interferon-γ release assay
- MTB:
-
Mycobacterium tuberculosis
- NAAT:
-
Nuclear acid amplification testing
- LASSO:
-
least absolute shrinkage operator
- ProPAED:
-
Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infections in children and adolescents study
- pTBred:
-
The Spanish Pediatric Tuberculosis Research Network
- ROC:
-
Receiver operating characteristic
- TB:
-
Tuberculosis
- TST:
-
Tuberculin skin test
- WHO:
-
World Health Organization
References
Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2010;50(Suppl 3):S184–94.
Nejat S, Buxbaum C, Eriksson M, Pergert M, Bennet R. Pediatric tuberculosis in Stockholm: a mirror to the world. Pediatr Infect Dis J. 2012;31(3):224–7.
Oesch Nemeth G, Nemeth J, Altpeter E, Ritz N. Epidemiology of childhood tuberculosis in Switzerland between 1996 and 2011. Eur J Pediatrics. 2014;173(4):457–62.
Oliwa JN, Karumbi JM, Marais BJ, Madhi SA, Graham SM. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Respir Med. 2015;3(3):235–43.
Steiner P, Rao M, Victoria MS, Jabbar H, Steiner M. Persistently negative tuberculin reactions: their presence among children with culture positive for Mycobacterium tuberculosis (tuberculin-negative tuberculosis). Am J Dis Child. 1980;134(8):747–50.
Sun L, Chen Y, Yi P, Yang L, Yan Y, Zhang K, et al. Serological detection of mycobacterium tuberculosis complex infection in multiple hosts by one universal ELISA. PLoS ONE. 2021;16(10):e0257920.
Vaezipour N, Fritschi N, Brasier N, Belard S, Dominguez J, Tebruegge M et al. Towards accurate point-of-care tests for tuberculosis in children. Pathogens. 2022 Mar 8;11(3):327.
WHO consolidated guidelines on tuberculosis. Geneva: World Health Organization; 2022.
Commercial Serodiagnostic Tests for Diagnosis of Tuberculosis. Policy Statement: World Health Organisation; 2011.
Neudecker Daniela PL, Tebruegge M, Lu L, Ritz N. Fritschi Nora. Serology to Diagnose Tuberculosis in Children - a narrative Review on Advances and current Performance. (unpublished).
Carpenter SM, Lu LL, Leveraging Antibody B. Cell and Fc receptor interactions to understand heterogeneous immune responses in tuberculosis. Front Immunol. 2022;13:830482.
Nziza N, Cizmeci D, Davies L, Irvine EB, Jung W, Fenderson BA, et al. Defining discriminatory antibody fingerprints in active and latent tuberculosis. Front Immunol. 2022;13:856906.
Lu LL, Das J, Grace PS, Fortune SM, Restrepo BI, Alter G. Antibody fc glycosylation discriminates between latent and active tuberculosis. J Infect Dis. 2020;222(12):2093–102.
Kissling M, Fritschi N, Baumann P, Buettcher M, Bonhoeffer J, Naranbhai V, et al. Monocyte, lymphocyte and neutrophil ratios - easy-to-use biomarkers for the diagnosis of pediatric tuberculosis. Pediatr Infect Dis J. 2023;42(6):520–7.
Meier NR, Sutter TM, Jacobsen M, Ottenhoff THM, Vogt JE, Ritz N. Machine learning algorithms evaluate immune response to novel mycobacterium tuberculosis antigens for diagnosis of tuberculosis. Front Cell Infect Microbiol. 2020;10:594030.
Hernanz-Lobo A, Noguera-Julian A, Minguell L, Lopez-Suarez A, Soriano-Arandes A, Espiau M, et al. Prevalence and clinical characteristics of children with nonsevere tuberculosis in Spain. Pediatr Infect Dis J. 2023;42(10):837–43.
Baer G, Baumann P, Buettcher M, Heininger U, Berthet G, Schafer J, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS ONE. 2013;8(8):e68419.
Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis. 2015;61Suppl(3):S179–87.
Tuberculosis in Switzerland- Guidance for healthcare professionals. Version 1.2021.
Baquero-Artigao F, Del Rosal T, Falcon-Neyra L, Ferreras-Antolin L, Gomez-Pastrana D, Hernanz-Lobo A, et al. Update on the diagnosis and treatment of tuberculosis. Pediatr (Engl Ed). 2023;98(6):460–9.
Cuevas LE, Browning R, Bossuyt P, Casenghi M, Cotton MF, Cruz AT, et al. Evaluation of tuberculosis diagnostics in children: 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S209–15.
Basu Roy R, Whittaker E, Seddon JA, Kampmann B. Tuberculosis susceptibility and protection in children. Lancet Infect Dis. 2019;19(3):e96–108.
Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Nelson LJ, et al. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8(3):278–85.
Seddon JA, Chiang SS, Esmail H, Coussens AK. The wonder years: what can primary school children teach us about immunity to mycobacterium tuberculosis? Front Immunol. 2018;9:2946.
Achkar JM, Ziegenbalg A. Antibody responses to mycobacterial antigens in children with tuberculosis: challenges and potential diagnostic value. Clin Vaccine Immunol. 2012;19(12):1898–906.
Araujo Z, Waard JH, Fernandez de Larrea C, Lopez D, Fandino C, Maldonado A, et al. Study of the antibody response against mycobacterium tuberculosis antigens in Warao Amerindian children in Venezuela. Mem Inst Oswaldo Cruz. 2004;99(5):517–24.
Dayal R, Sirohi G, Singh MK, Mathur PP, Agarwal BM, Katoch VM, et al. Diagnostic value of Elisa serological tests in childhood tuberculosis. J Trop Pediatr. 2006;52(6):433–7.
Narayana Y, Joshi B, Katoch VM, Mishra KC, Balaji KN. Differential B-cell responses are induced by mycobacterium tuberculosis PE antigens Rv1169c, Rv0978c, and Rv1818c. Clin Vaccine Immunol. 2007;14(10):1334–41.
Simonney N, Bourrillon A, Lagrange PH. Analysis of circulating immune complexes (CICs) in childhood tuberculosis: levels of specific antibodies to glycolipid antigens and relationship with serum antibodies. Int J Tuberc Lung Dis. 2000;4(2):152–60.
Kumar G, Dagur PK, Singh M, Yadav VS, Dayal R, Singh HB, et al. Diagnostic potential of Ag85C in comparison to various secretory antigens for childhood tuberculosis. Scand J Immunol. 2008;68(2):177–83.
Nonyane BAS, Nicol MP, Andreas NJ, Rimmele S, Schneiderhan-Marra N, Workman LJ, et al. Serologic responses in childhood pulmonary tuberculosis. Pediatr Infect Dis J. 2018;37(1):1–9.
Kasempimolporn S, Thaveekarn W, Kerdpanich P, Skulpichetrat U, Saekhow O, Boonchang S, et al. Performance of a rapid strip test for the serologic diagnosis of latent tuberculosis in children. J Clin Diagn Res. 2015;9(1):DC11–4.
Kasempimolporn S, Thaveekarn W, Promrungreang K, Khow O, Boonchang S, Sitprija V. Improved serodiagnostic sensitivity of strip test for latent tuberculosis. J Clin Diagn Res. 2017;11(6):DC01–3.
Awoniyi DO, Baumann R, Chegou NN, Kriel B, Jacobs R, Kidd M, et al. Detection of a combination of serum IgG and IgA antibodies against selected mycobacterial targets provides promising diagnostic signatures for active TB. Oncotarget. 2017;8(23):37525–37.
Brock M, Hanlon D, Zhao M, Pollock NR. Detection of mycobacterial lipoarabinomannan in serum for diagnosis of active tuberculosis. Diagn Microbiol Infect Dis. 2020;96(2):114937.
He H, Oka S, Han YK, Yamamura Y, Kusunose E, Kusunose M, et al. Rapid serodiagnosis of human mycobacteriosis by ELISA using cord factor (trehalose-6,6’-dimycolate) purified from mycobacterium tuberculosis as antigen. FEMS Microbiol Immunol. 1991;3(4):201–4.
Jones A, Pitts M, Al Dulayymi JR, Gibbons J, Ramsay A, Goletti D, et al. New synthetic lipid antigens for rapid serological diagnosis of tuberculosis. PLoS ONE. 2017;12(8):e0181414.
Maekura R, Okuda Y, Nakagawa M, Hiraga T, Yokota S, Ito M, et al. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J Clin Microbiol. 2001;39(10):3603–8.
Rens C, Chao JD, Sexton DL, Tocheva EI, Av-Gay Y. Roles for phthiocerol dimycocerosate lipids in mycobacterium tuberculosis pathogenesis. Microbiology (Reading). 2021 Mar;167(3).
Yan ZH, Yi L, Wei PJ, Jia HY, Wang J, Wang XJ, et al. Evaluation of panels of mycobacterium tuberculosis antigens for serodiagnosis of tuberculosis. Int J Tuberc Lung Dis. 2018;22(8):959–65.
Deng J, Bi L, Zhou L, Guo SJ, Fleming J, Jiang HW, et al. Mycobacterium tuberculosis proteome microarray for global studies of protein function and immunogenicity. Cell Rep. 2014;9(6):2317–29.
Ireton GC, Greenwald R, Liang H, Esfandiari J, Lyashchenko KP, Reed SG. Identification of mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol. 2010;17(10):1539–47.
Kunnath-Velayudhan S, Davidow AL, Wang HY, Molina DM, Huynh VT, Salamon H, et al. Proteome-scale antibody responses and outcome of mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J Infect Dis. 2012;206(5):697–705.
Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, et al. Dynamic antibody responses to the mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A. 2010;107(33):14703–8.
Li J, Wang Y, Yan L, Zhang C, He Y, Zou J, et al. Novel serological biomarker panel using protein microarray can distinguish active TB from latent TB infection. Microbes Infect. 2022;24(8):105002.
Li Z, Hu J, Liu P, Cui D, Di H, Wu S. Microarray-based selection of a serum biomarker panel that can discriminate between latent and active pulmonary TB. Sci Rep. 2021;11(1):14516.
Garay-Baquero DJ, White CH, Walker NF, Tebruegge M, Schiff HF, Ugarte-Gil C et al. Comprehensive plasma proteomic profiling reveals biomarkers for active tuberculosis. JCI Insight. 2020 Sep 17;5(18):e137427
Gupta S, Bhatia R, Datta KK. Serological diagnosis of childhood tuberculosis by estimation of mycobacterial antigen 60-specific immunoglobulins in the serum. Tuber Lung Dis. 1997;78(1):21–7.
Imaz MS, Comini MA, Zerbini E, Sequeira MD, Spoletti MJ, Etchart AA, et al. Evaluation of the diagnostic value of measuring IgG, IgM and IgA antibodies to the recombinant 16-kilodalton antigen of mycobacterium tuberculosis in childhood tuberculosis. Int J Tuberc Lung Dis. 2001;5(11):1036–43.
Raja A, Ranganathan UD, Bethunaickan R, Dharmalingam V. Serologic response to a secreted and a cytosolic antigen of mycobacterium tuberculosis in childhood tuberculosis. Pediatr Infect Dis J. 2001;20(12):1161–4.
Angeloni SDS, Dunbar S, Stone V, Swift S, xMAP. Cookbook: A collection of methods and protocols for developing multiplex assays with xMAP technology. 4th Edition ed: Luminex; 2018.
Lu LL, Smith MT, Yu KKQ, Luedemann C, Suscovich TJ, Grace PS, et al. IFN-gamma-independent immune markers of mycobacterium tuberculosis exposure. Nat Med. 2019;25(6):977–87.
Schlottmann SA, Jain N, Chirmule N, Esser MT. A novel chemistry for conjugating pneumococcal polysaccharides to luminex microspheres. J Immunol Methods. 2006;309(1–2):75–85.
Grover A, Wang E, Zweig A, Ermon S. Stochastic optimization of sorting networks via continuous relaxations. arXiv:190308850. 2019.
Xie SM, Ermon S. Reparameterizable Subset Sampling via Continuous Relaxations. Proceedings of the 28th International Joint Conference on Artificial Intelligence; Macao, China: AAAI Press; 2019. pp. 3919–25.
Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–43. e14.
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–44.
Hermann C, King CG. TB or not to be: what specificities and impact do antibodies have during tuberculosis? Oxf Open Immunol. 2021;2(1).
Brennan PJ. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis. 1989;11(Suppl 2):S420–30.
Garcia-Vilanova A, Chan J, Torrelles JB. Underestimated manipulative roles of mycobacterium tuberculosis cell envelope glycolipids during infection. Front Immunol. 2019;10:2909.
Lyashchenko K, Colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro ML. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66(8):3936–40.
Wu X, Yang Y, Zhang J, Li B, Liang Y, Zhang C, et al. Humoral immune responses against the mycobacterium tuberculosis 38-kilodalton, MTB48, and CFP-10/ESAT-6 antigens in tuberculosis. Clin Vaccine Immunol. 2010;17(3):372–5.
Zimmermann N, Thormann V, Hu B, Kohler AB, Imai-Matsushima A, Locht C, et al. Human isotype-dependent inhibitory antibody responses against mycobacterium tuberculosis. EMBO Mol Med. 2016;8(11):1325–39.
Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 2011;186(5):3113–9.
Acknowledgements
We thank the local Principal Investigators of the CITRUS study: Sara Bernhard, Lisa Kottanattu, Andrea Duppenthaler, Anne Morand, Jürg Barben, Christoph Berger, Christa Relly, Isabelle Rochat, Marie Rohr, as well as of Noemi Meier and Andrea Marten for their contribution to plasma sample collection. Our gratitude extends to the investigators of the ProPAED study: Gurli Baer, Jan Bonhoeffer, Philipp Baumann, Michael Buettcher, Ulrich Heininger, Gerald Berthet, Julia Schäfer, Heiner Bucher, Daniel Trachsel, Jaques Schneider, Muriel Gambon, Diana Reppucci, Jessica Bonhoeffer, Jody Stähelin-Massik, Philipp Schuetz, Beat Mueller, Gabor Szinnai, and Urs Schaad. We also appreciate the efforts of the recruiters of the pTBred network: Mar Santos Sebastián, Marisa Navarro, Elena Rincón, Jesús Saavedra, David Aguilera, and the laboratory and biobank manager Andrea López Suarez. Special thanks go to the children and their parents for their essential participation in this study.
Funding
The CITRUS study is supported by grant from: Lunge Zürich, Bangerter Rhyner Stiftung, Swiss Lung Association, Rozalia Foundation, Draksler Foundation, Nora van Meeuwen-Häfliger Foundation. NR was supported by the University of Basel academic mid-level faculty grant. DN, NF and NR were supported by the Thomi Hopf Foundation. TS is supported by the grant #2021 − 911 of the Strategic Focal Area “Personalized Health and Related Technologies (PHRT)” of the ETH Domain (Swiss Federal Institutes of Technology). PTBred received funding to conduct this project by a competitive grant from Instituto de Salud Carlos III through the projects PI17/00711 and PI20/01607, co-financed by the European Regional Development’s funds (FEDER). The Division of Infectious Diseases and Vaccines, University Children’s Hospital, Basel, Switzerland supported the ProPAED study as an investigator-initiated trial. Lenette Lu is supported by NIH (5R01AI158858) and UTSW Disease Oriented Clinical Scholars Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Open access funding provided by University of Basel
Author information
Authors and Affiliations
Contributions
This study protocol was designed by DN, NF, LL, PL, TS, BS, MT, and NR; all authors reviewed and revised the protocol and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Ethikkommission Nordwestschweiz (ref: EKNZ 2016 − 01094) for the CITRUS study, by the Ethics Committee of Basel (ref: EKBB 369/08) for the ProPAED study, and by the Gregorio Marañón Ethics Committee (code 359/21) for the pTBred network. Written informed consent to participate in this study was provided by the legal guardian or next of kin of the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Following publication of the original article, we have been notified that due to a system error, the proofs were not sent to the corresponding author, so they were not able to submit their corrections.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Neudecker, D., Fritschi, N., Sutter, T. et al. Evaluation of serological assays for the diagnosis of childhood tuberculosis disease: a study protocol. BMC Infect Dis 24, 481 (2024). https://doi.org/10.1186/s12879-024-09359-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09359-0