- Research
- Open access
- Published:
Systematic review and meta-analysis of hepatitis E seroprevalence in Southeast Asia: a comprehensive assessment of epidemiological patterns
BMC Infectious Diseases volume 24, Article number: 525 (2024)
Abstract
The burden of hepatitis E in Southeast Asia is substantial, influenced by its distinct socio-economic and environmental factors, as well as variations in healthcare systems. The aim of this study was to assess the pooled seroprevalence of hepatitis E across countries within the Southeast Asian region by the UN division.
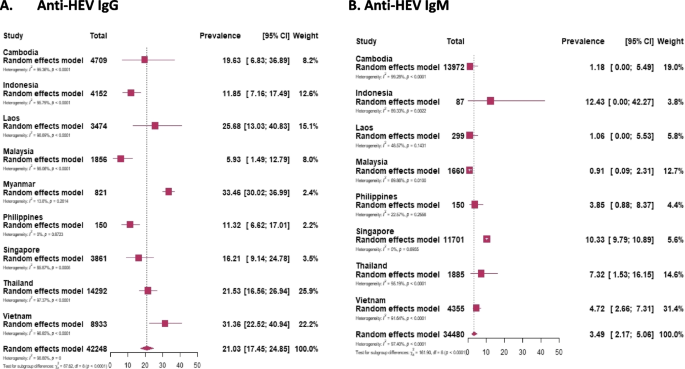
The study analyzed 66 papers across PubMed, Web of Science, and Scopus databases, encompassing data from of 44,850 individuals focusing on anti-HEV seroprevalence. The investigation spanned nine countries, excluding Brunei and East Timor due to lack of data. The pooled prevalence of anti-HEV IgG was determined to be 21.03%, with the highest prevalence observed in Myanmar (33.46%) and the lowest in Malaysia (5.93%). IgM prevalence was highest in Indonesia (12.43%) and lowest in Malaysia (0.91%). The study stratified populations into high-risk (farm workers, chronic patients) and low-risk groups (general population, blood donors, pregnant women, hospital patients). It revealed a higher IgG—28.9%, IgM—4.42% prevalence in the former group, while the latter group exhibited figures of 17.86% and 3.15%, respectively, indicating occupational and health-related vulnerabilities to HEV.
A temporal analysis (1987–2023), indicated an upward trend in both IgG and IgM prevalence, suggesting an escalating HEV burden.
These findings contribute to a better understanding of HEV seroprevalence in Southeast Asia, shedding light on important public health implications and suggesting directions for further research and intervention strategies.
Key points
Research Question
Investigate the seroprevalence of hepatitis E virus (HEV) in Southeast Asian countries focusing on different patterns, timelines, and population cohorts.
Findings
Sporadic Transmission of IgG and IgM Prevalence:
• Pooled anti-HEV IgG prevalence: 21.03%
• Pooled anti-HEV IgM prevalence: 3.49%
Seroprevalence among specific groups:
High-risk group (farm workers and chronic patients):
• anti-HEV IgG: 28.9%
• anti-HEV IgM: 4.42%
Low-risk group (general population, blood donors, pregnant women, hospital patients):
• anti-HEV IgG: 17.86%
• anti-HEV IgM: 3.15%
Temporal Seroprevalence of HEV:
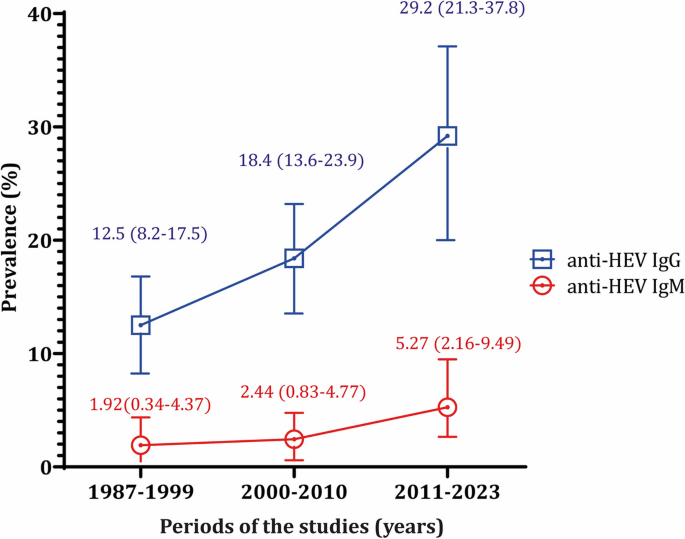
Anti-HEV IgG prevalence increased over decades (1987–1999; 2000–2010; 2011–2023): 12.47%, 18.43%, 29.17% as an anti-HEV IgM prevalence: 1.92%, 2.44%, 5.27%
Importance
Provides a comprehensive overview of HEV seroprevalence in Southeast Asia.
Highlights variation in seroprevalence among different population groups.
Reveals increasing trend in HEV seroprevalence over the years.
Distinguishes between sporadic and epidemic cases for a better understanding of transmission dynamics.
Introduction
Hepatitis E is a major global health concern caused by the hepatitis E virus (HEV), which is a small, nonenveloped, single-stranded, positive-sense RNA virus belonging to the Paslahepevirus genus in the Hepeviridae family. There are eight genotypes of HEV: HEV-1 and HEV-2 infect only humans, HEV-3, HEV-4, and HEV-7 infect both humans and animals, while HEV-5, HEV-6, and HEV-8 infect only animals [1].
HEV infections affect millions of people worldwide each year, resulting in a significant number of symptomatic cases and deaths. In 2015, the World Health Organization (WHO) reported approximately 44,000 deaths from hepatitis E, accounting for 3.3% of overall mortality attributed to viral hepatitis [2]. The primary mode of transmission for hepatitis E is through the fecal–oral route. Outbreaks of the disease are often associated with heavy rainfall and flooding [3, 4]. Additionally, sporadic cases can occur due to poor sanitation, vertical transmission, blood transfusion or close contact with infected animals, which serve as hosts for the virus [5]. Southeast Asia carries a substantial burden of hepatitis E, influenced by its unique socio-economic and environmental factors as well as variations in healthcare systems. Understanding the seroprevalence of hepatitis E in this region is crucial for implementing targeted public health interventions and allocating resources. To achieve the effective control and prevention of HEV, it is required to address the waterborne transmission and considering the specific characteristics of each region. By taking these measures, healthcare authorities can work towards reducing the global impact of hepatitis E on public health. Systematic reviews and meta-analyses on hepatitis E play a crucial role in synthesizing and integrating existing research findings, providing comprehensive insights into the epidemiology, transmission, and burden of the disease, thereby aiding evidence-based decision-making and public health strategies [6, 7].
Recent systematic reviews and meta-analysis conducted on hepatitis E have varied in their scope or were limited by a smaller number of source materials [8, 9]. The objective of this study was to determine the pooled seroprevalence of hepatitis E in countries within Southeast Asia by aggregating findings from a multitude of primary studies conducted across the region.
Methods
To commence this systematic review and meta-analysis, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and used the PRISMA assessment checklist [Supplementary Table 1]. The study included pertinent research conducted within the population of Southeast Asian countries, as outlined by the United Nations [10], and perform a meta-analysis on the seroprevalence of hepatitis E in this specific region.
PICOT assessment
Population
In this systematic review and meta-analysis, the eligible population comprised individuals from the Southeast Asia region, irrespective of age, gender, ethnic characteristics, or specific chronic diseases. However, studies involving populations outside the designated countries, travelers, migrants, animal species studies, and those lacking clear descriptions of the study population were excluded.
Intervention and comparison
Intervention and comparison are not applicable to the prevalence studies.
Outcome
Anti-HEV antibodies positivity either total antibodies or IgG or IgM among the Southeast Asian countries' population was assessed.
Time frame
All studies conducted between 1987 and 2023 were included in this meta-analysis.
Search strategy
To conduct the data search, we utilized three databases, namely “PubMed”, “Scopus”, and “Web of Science”. The search terms comprised keywords related to the Hepatitis E virus, such as “Hepatitis E virus” OR “Hepatitis E” OR “HEV” AND names of each country “Brunei”, “Cambodia”, “Timor-Leste” OR “East-Timor”, “Laos” OR “Lao PDR”, “Indonesia”, “Malaysia”, “Myanmar” OR “Burma”, “Philippines”, “Singapore”, “Thailand”, “Vietnam” and “Southeast Asia”.
The search process in the databases finished on May 29th, 2023, with two members of the study team conducting independent searches. Subsequently, the search results were unified. A grey literature search was performed from June 25th to 30th, 2023, by examining the references of review manuscripts and conference materials, along with using specific keywords in the Google Scholar database. Notably, during the gray literature search, additional studies from the Philippines that were initially missing in the first search were identified and included. Moreover, due to the diverse language expertise of the team, studies in Russian and French related to Cambodia and Vietnam were also considered for inclusion.
After applying the inclusion and exclusion criteria, each article selected for this systematic review (SR) was considered relevant. The quality assessment of each article was conducted using specific JBI critical appraisal instruments [11] [Supplementary Table 2].
Sporadic transmission of HEV infection
For the systematic review and meta-analysis of sporadic infection of HEV, we divided the study population into cohorts by countries, by risk of acquiring HEV—low and high risk. The low risk cohort included the general population (apparently healthy individuals, students, some ethnic populations, or individuals included in original studies as “general population”), blood donors, pregnant women, and hospital patients, while pig farmers, those with chronic hepatitis, HIV positive patients, and solid organ transplant patients in the high-risk group.
Lastly, we analyzed data in three decades—1987–1999, 2000–2010, and 2011–2023—to reveal seroprevalence rates over time.
Epidemic outbreaks of HEV infection
We separated epidemic outbreaks from sporadic cases due to distinct patterns and scale of transmission in epidemy. Epidemics are characterized by rapid and widespread transmission, affecting a large population within a short period and often following a specific pattern or route of propagation.
Statistical analysis
A meta-analysis of proportions was conducted using the 'meta' and 'metafor' packages in the R statistical software. To account for small proportions, the Freeman-Tukey double arcsine method was applied to transform the data. The Dersimonian and Laird method, which employs a random-effects model, was utilized for the meta-analysis, and the results were presented in a forest plot. Confidence intervals (CIs) for the proportions of individual studies were computed using the Clopper-Pearson method.
Heterogeneity was evaluated using the Cochran Q test and quantified by the I2 index. Heterogeneity was considered significant if the p-value of the Cochran Q test was below 0.05.
For the assessment of publication bias, a funnel plot displaying the transformed proportions against the sample size was created. The symmetry of the plot was examined using the Egger test (p < 0.1).
Results
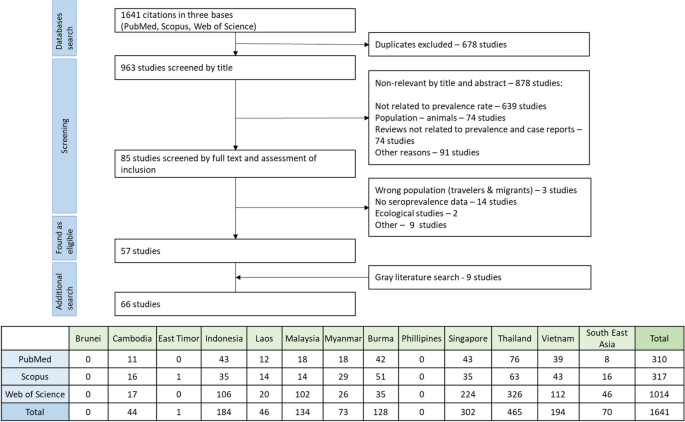
The initial search yielded 1641 articles, which covered 9 out of 11 Southeast Asia countries. We couldn't find any information on hepatitis E from Brunei. We excluded a study from East Timor because it focused on the wrong population (US Army troops). The final screening resulted in the selection of 57 relevant studies, and the grey literature search added 9 more papers that met our inclusion criteria (Fig. 1). Among 9 papers through a grey literature, two relevant studies from the Philippines [12, 13], one each from Indonesia [14] and Lao PDR [15], one study covered both Vietnam and Cambodia [16], one study provided HEV seroepidemiology information for Myanmar, Thailand, and Vietnam [17], two studies reported in Russian [18, 19] (from Vietnam) and one reported in French [16] (from Vietnam and Cambodia). In total, our analysis included 66 papers from which we extracted data. This involved a total of 44,850 individuals (Table 1).
Sporadic transmission IgG and IgM prevalence in Southeast Asian countries (excluding outbreak settings)
The sporadic cases involving 42,248 participants out of 44,850 participants (the remaining 2,602 people are considered in the “Epidemic outbreaks” section) from Southeast Asian countries the pooled prevalence of IgG was found to be 21.03%, while for IgM, it was 3.49% among 34,480 individuals who were tested (Fig. 2). Among these countries, Myanmar registered the highest pooled prevalence of IgG at 33.46%, while Malaysia had the lowest at 5.93%. For IgM prevalence, Indonesia had the highest rate at 12.43%, and Malaysia again had the lowest at 0.91% (Table 2) [Supplementary Figures 1 and 6].
Seroprevalence among specific groups
High risk of acquiring HEV
The high-risk group, which included farm workers and chronic patients, demonstrated a pooled anti-HEV IgG prevalence of 28.9%, with IgM prevalence at 4.42% [Supplementary Figures 2 and 8].
Chronic patients
This group, comprising individuals with chronic liver disease, HIV infection, or solid organ transplantation, exhibited the highest prevalence of pooled IgG among all cohorts, standing at 29.2%. Additionally, IgM prevalence was 3.9% [Supplementary Figures 2 and 7].
Farm workers
Farm workers were divided into several subgroups based on exposure to animals (reservoirs of HEV), including pig or ruminant farmers, slaughterhouse workers, butchers, and meat retailers. Among this group, the highest IgG prevalence was observed at 28.4%, while the pooled IgM level was 6.21% [Supplementary Figures 2 and 7].
Low risk of acquiring HEV
The low-risk group, comprising the general population, blood donors, pregnant women, and hospital patients, exhibited anti-HEV IgG and IgM prevalence of 17.86% and 3.15%, respectively. [Supplementary Figures 2 and 9].
General population
The general population in Southeast Asian countries, represented by 22,571 individuals, showed a presence of IgG in 21.4% of them. IgM was tested in 10,304 participants, and 2.63% of acute infection cases were identified [Supplementary Figures 2 and 7].
Blood donors
Blood donors, as a selected subgroup of the general population, exhibit differences in health status, age, gender distribution, and representativeness, warranting separate assessment. Among blood donors in Southeast Asian countries, the pooled prevalence of IgG and IgM were found to be 11.77% and 0.83%, respectively [Supplementary Figures 2 and 7].
Pregnant women
Pregnant women considered a vulnerable group regarding disease consequences, demonstrated an anti-HEV IgG prevalence of 18.56% among 1,670 individuals included in the study. Furthermore, 1.54% of them tested positive for anti-HEV IgM [Supplementary Figures 2 and 7].
Hospital patients
A group of 18,792 patients who visited hospitals with clinical signs of acute infection, jaundice, high temperature, and elevated liver enzymes, showed anti-HEV IgG and IgM prevalence of 16.3% and 4.45%, respectively [Supplementary Figures 2 and 7].
Temporal seroprevalence of HEV
Given the studies' long duration, the data was presented by decades: 1987–1999, 2000–2010, and 2011–2023. The prevalence of IgG showed an upward trend over these decades, with rates of 12.47%, 18.43%, and 29.17%. Similarly, for IgM, the prevalence rates were 1.92%, 2.44%, and 5.27% for the first, second, and third decades, respectively (Fig. 3).
Evaluating the trend of seroprevalence over decades within the same population and country proved challenging due to the limited availability of research papers. Consequently, we assessed anti-HEV antibody prevalence over decades, considering population cohorts and individual countries.
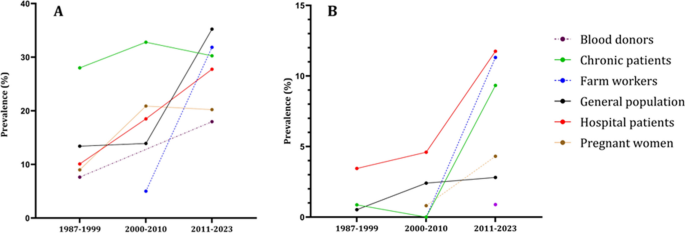
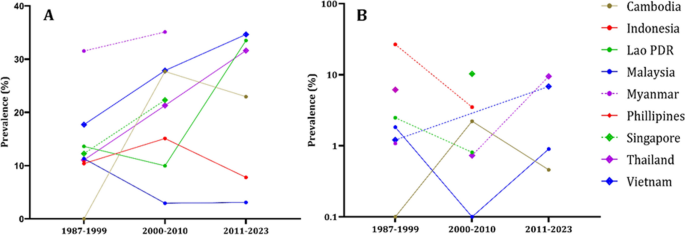
In Fig. 4, we can see that all population groups show a consistent increase in the prevalence of both IgG and IgM antibodies over the decades. Figure 5, we analyze the prevalence of anti-HEV antibodies in different countries over time, except for Indonesia and Malaysia, where we observe an increase in prevalence.
The epidemiological data regarding the occurrence of anti-HEV IgG (A) and anti-HEV IgM (B) antibodies within population cohorts across Southeast Asian nations divided by decades. The population cohorts delineated by the disrupted lines in the figure lack comprehensive data representation, as they provide information for only two out of three decades. Blood donors group has the anti-HEV IgM only for the last decade
The epidemiological data regarding the occurrence of anti-HEV IgG (A) and anti-HEV IgM (B) antibodies within countries of Southeast Asia divided by decades. The countries delineated by the disrupted lines in the figure lack comprehensive data representation, as they provide information for only two out of three decades. Philippines has the anti-HEV IgG antibodies information only for the first decade. Philippines, Myanmar, Singapore have anti-HEV IgM information only for single decade
Some studies lacked information on the collection time of the samples [13, 19, 41, 48, 59, 62, 64, 82]. In these studies, the pooled IgG and IgM prevalence was 26.5% and 4.75%, respectively [Supplementary Figures 3, 4, 5, 10, 11, 12].
Epidemic outbreaks
We separated epidemic outbreaks from sporadic cases due to distinct patterns and scale of transmission in epidemy. Epidemics are characterized by rapid and widespread transmission, affecting a large population within a short period and often following a specific pattern or route of propagation. The outbreaks occurred between 1987 and 1998 in several Southeast Asian countries, namely Indonesia [31, 33, 34], Vietnam [77], and Myanmar [54] [Supplementary Figure 13]. These outbreak investigations involved a total of 2,602 individuals, with most participants from Indonesia (2,292 individuals). The studies were mainly conducted using a case–control design. Among the participants, 876 were considered controls, while 1,726 were classified as cases. The pooled prevalence of total anti-HEV immunoglobulins was estimated as 61.6% (95% CI 57.1–66) (Table 2).
Assessment of publication bias
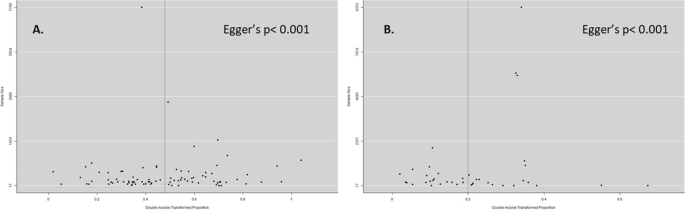
We checked for publication bias using a funnel plot and Egger's test. Both the studies on anti-HEV IgG and IgM showed asymmetry with Egger's test indicating a p-value less than 0.001 for both cases (Fig. 6).
Discussion
A paper search yielded varying numbers of manuscripts from Southeast Asian countries. The Philippines had the fewest studies, while Thailand had the highest with 15 studies. No data was found for Brunei Darussalam and East Timor or Timor Leste on the human species.
The results of this study provide valuable insights into the seroprevalence of IgG and IgM antibodies against HEV in different populations across Southeast Asian countries. Understanding the prevalence of these antibodies is essential for assessing the burden of HEV infection and identifying high-risk groups.
The extensive analysis of anti-HEV IgG prevalence in this study covered a wide range of population groups in Southeast Asia, including the general population, blood donors, pregnant women, hospital patients, farm workers, and chronic patients. The results unveiled an overall pooled prevalence of 21.03%, indicating significant exposure to the Hepatitis E virus among individuals in the region at some point in their lives. Moreover, a consistent increase in IgG prevalence was observed over the years, with the highest prevalence occurring in the most recent decade (2011–2023). This suggests a progressive rise in HEV exposure within the region.
Upon examining the prevalence data across different decades and population cohorts, a uniform upward trend in HEV antibody prevalence became apparent across all groups. Several factors could be assessed as potential contributors to this trend:
Notably, the expanding population in Southeast Asian nations during this timeframe increased the number of individuals at risk of Hepatitis E infection.
The rapid urbanization, characterized by the migration from rural to urban areas, led to higher population density and conditions conducive to Hepatitis E virus transmission [84]. Access to clean drinking water and adequate sanitation facilities emerged as critical factors in preventing Hepatitis E. Regions with inadequate infrastructure, particularly in water and sanitation, faced an elevated risk due to contaminated water sources. Climate-related events, such as heavy rainfall and flooding, significantly impacted waterborne diseases like Hepatitis E. The increasing frequency and severity of such events emphasized the importance of considering climate-related factors in assessing prevalence trends [85]. Consumption of contaminated or undercooked meat, particularly pork, was identified as a source of Hepatitis E transmission. Changes in food consumption habits over time may have contributed to changes in seroprevalence [86]. Limited access to healthcare facilities in certain areas exacerbated the spread of Hepatitis E. Increased awareness together with advances in medical research and the establishment of robust surveillance systems likely improved the detection and reporting of Hepatitis E cases, contributing to the observed increase in seroprevalence [87,88,89]. These multifaceted factors have likely played a collective role in shaping the changing landscape of Hepatitis E seroprevalence in Southeast Asian nations over the past decades. The upward trend emphasizes the importance of continued monitoring, intervention, and public health measures to mitigate the spread of Hepatitis E in the region.
Among specific populations, pregnant women exhibited an IgG prevalence of 18.56%, indicating that a considerable number of pregnant individuals have been exposed to HEV. Pregnant women are particularly vulnerable to the consequences of HEV infection, as it can lead to severe outcomes for both the mother and the foetus.
Hospital patients with clinical signs of acute infection showed an IgG prevalence of 16.3%, suggesting that HEV is still a significant cause of acute hepatitis cases in the hospital setting. Similarly, farm workers, especially those exposed to animals (reservoirs of HEV), had a high prevalence of IgG (28.4%), highlighting the occupational risk associated with zoonotic transmission.
Chronic patients, including individuals with chronic liver disease, HIV infection, or solid organ transplantation, exhibited the highest pooled IgG prevalence among all cohorts at 29.2%. This finding underscores the importance of monitoring HEV infection in immunocompromised individuals, as they may develop chronic HEV infection, which can lead to severe liver complications.
The prevalence of IgM antibodies, which are indicative of recent or acute HEV infection, was lower overall compared to IgG. The general population showed an IgM prevalence of 2.63% among acute infection cases. Among hospital patients exhibiting clinical signs of acute infection, the prevalence of IgM antibodies indicative of recent or acute HEV infection was higher at 4.45%.
Farm workers, particularly those exposed to animals, demonstrated the highest IgM prevalence at 6.21%. This finding highlights the occupational risk of acquiring acute HEV infection in this population due to direct or indirect contact with infected animals.
The study also identified a high-risk group, consisting of farm workers and chronic patients, with a pooled IgG prevalence of 28.9% and an IgM prevalence of 4.42%. This group is particularly susceptible to HEV infection and requires targeted interventions to reduce transmission and prevent severe outcomes.
Overall, this study provides valuable data on the seroprevalence of HEV antibodies in different populations in Southeast Asian countries. It highlights the importance of continued surveillance and public health interventions to control HEV transmission, especially in vulnerable groups. Understanding the prevalence trends over time can aid in developing effective strategies for the prevention and management of HEV infections in the region. However, further research and studies are warranted to explore the underlying factors contributing to the observed seroprevalence trends and to design targeted interventions to reduce HEV transmission in specific populations. Among the countries of Southeast Asia Myanmar was the most for HEV infection, while Malaysia registered the lowest seroprevalence.
This study has some limitations that we should be aware of. We looked at studies in three languages (English, Russian, and French), but we couldn't find data from two out of the 11 countries. This means we might not have a complete picture of the disease's prevalence in the whole region.
The way we divided the groups based on occupation or status could be questioned. Different criteria might give us different results, so it's something we need to consider. Another challenge is that the study covers a long time from 1989 to 2023 by published research and involves many different countries. This makes it difficult to compare the results because the tests used, and the diagnostic abilities might have changed over time and vary across countries.
Despite these limitations, our study presents a detailed epidemiologic report of combined seroprevalence data for HEV in Southeast Asian countries following the UN division. It gives us a basic understanding of the disease's prevalence in the region and offers some insights into potential risk factors. However, to get a more accurate picture, future research should address these limitations and include data from all countries in the region. Furthermore, certain countries such as Myanmar and the Philippines have not reported HEV prevalence data since 2006 and 2015, respectively. The absence of recent HEV prevalence reports from certain countries raises concerns about the availability of up-to-date epidemiological data for assessing the current status of hepatitis E virus infections in these regions.
Conclusion
Our comprehensive analysis study involving Southeast Asian countries provides significant insights into the seroprevalence of hepatitis E virus (HEV) infection in this region and in various populations. The rates of anti-HEV antibodies observed among different groups, as well as the increasing trend in seroprevalence over decades, emphasize the dynamic nature of HEV transmission in the region. These findings contribute to a better understanding of HEV prevalence across countries, populations, and time periods in Southeast Asia, shedding light on important public health implications and suggesting directions for further research and intervention strategies.
Availability of data and materials
All data generated or analyzed during this study were included in this paper either in the results or supplementary information.
Abbreviations
- HEV:
-
Hepatitis E Virus
- PRISMA:
-
Preferred reporting items for systematic review and meta-analysis
- ELISA:
-
Enzyme-Linked Immunosorbent Essay
- HEV IgG:
-
Hepatitis E virus Immunoglobulin G
- HEV IgM:
-
Hepatitis E Virus Immunoglobulin M
References
Smith DB, Izopet J, Nicot F, Simmonds P, Jameel S, Meng XJ, et al. Update: proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J Gen Virol. 2020 [cited 2023 Aug 3];101(7):692. Available from: /pmc/articles/PMC7660235/.
Hepatitis E. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e. Accessed 22 July 2023.
Viswanathan R. A review of the literature on the epidemiology of infectious hepatitis. Indian J Med Res. 1957;45:145–55.
Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992 [cited 2023 Jul 20];70(5):597. Available from: /pmc/articles/PMC2393368/?report=abstract.
Aslan AT, Balaban HY. Hepatitis E virus: epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020;26(37):5543–60.
Mulrow CD. Rationale for systematic reviews. BMJ. 1994 [cited 2023 Jul 20];309(6954):597–9. Available from: https://pubmed.ncbi.nlm.nih.gov/8086953/.
Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376–80.
Wasuwanich P, Thawillarp S, Ingviya T, Karnsakul W. Hepatitis E in Southeast Asia. Siriraj Med J. 2020 [cited 2023 Jul 20];72(3):259–64. Available from: https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/240129.
Raji YE, Peck Toung O, Mohd N, Zamberi T, Sekawi B, MohdTaib N, et al. A systematic review of the epidemiology of hepatitis E virus infection in South – Eastern Asia. Virulence. 2021 [cited 2023 Jul 20];12(1):114. Available from: /pmc/articles/PMC7781573/.
South East Asia. Available from: https://www.unep.org/ozonaction/south-east-asia. Accessed 28 May 2023.
Chapter 5: Systematic reviews of prevalence and incidence - JBI Manual for Evidence Synthesis - JBI Global Wiki. [accessed 2023 May 20]. Available from: https://jbi-global-wiki.refined.site/space/MANUAL/4688607/Chapter+5%3A+Systematic+reviews+of+prevalence+and+incidence.
Gloriani-Barzaga N, Cabanban A, Graham RR, Florese RH. Hepatitis E virus infection diagnosed by serology: a report of cases at the San Lazaro Hospital Manila. Phil J Microbiol Infect Dis. 1997;26(4):169–72.
Lorenzo AA, De Guzman TS, Su GLS. Detection of IgM and IgG antibodies against hepatitis E virus in donated blood bags from a national voluntary blood bank in Metro Manila Philippines. Asian Pac J Trop Dis. 2015;5(8):604–5.
Jennings GB, Lubis I, Listiyaningsih E, Burans JP, Hyams KC. Hepatitis E virus in Indonesia. Trans R Soc Trop Med Hyg. 1994 [cited 2023 Jul 24];88(1):57. Available from: https://pubmed.ncbi.nlm.nih.gov/8154003/.
Pauly M, Muller CP, Black AP, Snoeck CJ. Intense human-animal interaction and limited capacity for the surveillance of zoonoses as drivers for hepatitis E virus infections among animals and humans in Lao PDR. Int J Infect Dis. 2016 [cited 2023 Jul 24];53:18. Available from: http://www.ijidonline.com/article/S1201971216312693/fulltext.
Buchy P, Monchy D, An TT, Srey CT, Tri DV, Son S, Glaziou P, Chien BT. Prévalence de marqueurs d’infection des hépatites virales A, B, C et E chez des patients ayant une hypertransaminasémie a Phnom Penh (Cambodge) et Nha Trang (Centre Vietnam) [Prevalence of hepatitis A, B, C and E virus markers among patients with elevated levels of Alanine aminotransferase and Aspartate aminotransferase in Phnom Penh (Cambodia) and Nha Trang (Central Vietnam)]. Bull Soc Pathol Exot. 2004;97(3):165–71.
Abe K, Li T, Ding X, Win KM, Shrestha PK, Quang VX, Ngoc TT, Taltavull TC, Smirnov AV, Uchaikin VF, Luengrojanakul P. International collaborative survey on epidemiology of hepatitis E virus in 11 countries. Southeast Asian J Trop Med Public Health. 2006;37(1):90–5.
Lichnaia EV, Pham THG, Petrova OA, Tran TN, Bui TTN, Nguyen TT, et al. Hepatitis e virus seroprevalence in indigenous residents of the Hà Giang northern province of Vietnam. Russ J Infect Immun. 2021;11(4):692–700.
Ostankova YuV, Semenov AV, Valutite DE, Zueva EB, Serikova EN, Shchemelev AN, et al. Enteric viral hepatitis in the Socialist Republic of Vietnam (Southern Vietnam). Jurnal Infektologii. 2021;13(4):72–8. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85123451810&doi=10.22625%2f2072-6732-2021-13-4-72-78&partnerID=40&md5=968b7e50231d40b12aded0e0da133936.
Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, et al. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86(2):246–53. Available from: https://pubmed.ncbi.nlm.nih.gov/22302857/.
Nouhin J, Madec Y, Prak S, Ork M, Kerleguer A, Froehlich Y, et al. Declining hepatitis E virus antibody prevalence in Phnom Penh, Cambodia during 1996–2017. Epidemiol Infect. 2018;147:e26. Available from: https://pubmed.ncbi.nlm.nih.gov/30309396/.
Nouhin J, Prak S, Madec Y, Barennes H, Weissel R, Hok K, et al. Hepatitis E virus antibody prevalence, RNA frequency, and genotype among blood donors in Cambodia (Southeast Asia). Transfusion (Paris). 2016;56(10):2597–601. Available from: https://pubmed.ncbi.nlm.nih.gov/27480100/.
Nouhin J, Barennes H, Madec Y, Prak S, Hou SV, Kerleguer A, et al. Low frequency of acute hepatitis E virus (HEV) infections but high past HEV exposure in subjects from Cambodia with mild liver enzyme elevations, unexplained fever or immunodeficiency due to HIV-1 infection. J Clin Virol. 2015;71:22–7. Available from: https://pubmed.ncbi.nlm.nih.gov/26370310/.
Yamada H, Takahashi K, Lim O, Svay S, Chuon C, Hok S, et al. Hepatitis E Virus in Cambodia: prevalence among the general population and complete genome sequence of genotype 4. PLoS One. 2015;10:e0136903. Available from: https://pubmed.ncbi.nlm.nih.gov/26317620/.
Chhour YM, Ruble G, Hong R, Minn K, Kdan Y, Sok T, et al. Hospital-based diagnosis of hemorrhagic fever, encephalitis, and hepatitis in Cambodian children. Emerg Infect Dis. 2002;8(5):485–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2732496/pdf/01-0236-FinalR.pdf.
Utsumi T, Hayashi Y, Lusida MI, Amin M, Soetjipto, Hendra A, et al. Prevalence of hepatitis E virus among swine and humans in two different ethnic communities in Indonesia. Arch Virol. 2011;156(4):689–93. Available from: https://pubmed.ncbi.nlm.nih.gov/21191625/.
Mizuo H, Suzuki K, Takikawa Y, Sugai Y, Tokita H, Akahane Y, et al. Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J Clin Microbiol. 2002 [cited 2023 Jul 24];40(9):3209. Available from: /pmc/articles/PMC130758/.
Achwan WA, Muttaqin Z, Zakaria E, Depamede SA, Mulyanto, Sumoharjo S, et al. Epidemiology of hepatitis B, C, and E viruses and human immunodeficiency virus infections in Tahuna, Sangihe-Talaud Archipelago Indonesia. Intervirology. 2007;50(6):408–11. Available from: https://pubmed.ncbi.nlm.nih.gov/18185013/.
Surya IG, Kornia K, Suwardewa TG, Mulyanto, Tsuda F, Mishiro S. Serological markers of hepatitis B, C, and E viruses and human immunodeficiency virus type-1 infections in pregnant women in Bali Indonesia. J Med Virol. 2005;75(4):499–503. Available from: https://pubmed.ncbi.nlm.nih.gov/15714491/.
Wibawa ID, Suryadarma IG, Mulyanto, Tsuda F, Matsumoto Y, Ninomiya M, et al. Identification of genotype 4 hepatitis E virus strains from a patient with acute hepatitis E and farm pigs in Bali Indonesia. J Med Virol. 2007;79(8):1138–46. Available from: https://pubmed.ncbi.nlm.nih.gov/17596841/.
Sedyaningsih-Mamahit ER, Larasati RP, Laras K, Sidemen A, Sukri N, Sabaruddin N, et al. First documented outbreak of hepatitis E virus transmission in Java, Indonesia. Trans R Soc Trop Med Hyg. 2002;96(4):398–404. Available from: https://pubmed.ncbi.nlm.nih.gov/12497976/.
Widasari DI, Yano Y, Utsumi T, Heriyanto DS, Anggorowati N, Rinonce HT, et al. Hepatitis E virus infection in two different regions of Indonesia with identification of swine HEV genotype 3. Microbiol Immunol. 2013;57(10):692–703. Available from: https://pubmed.ncbi.nlm.nih.gov/23865729/.
Corwin A, Putri MP, Winarno J, Lubis I, Suparmanto S, Sumardiati A, et al. Epidemic and sporadic hepatitis E virus transmission in West Kalimantan (Borneo). Indonesia Am J Trop Med Hyg. 1997;57(1):62–5. Available from: https://pubmed.ncbi.nlm.nih.gov/9242320/.
Corwin A, Jarot K, Lubis I, Nasution K, Suparmawo S, Sumardiati A, et al. Two years’ investigation of epidemic hepatitis E virus transmission in West Kalimantan (Borneo), Indonesia. Trans R Soc Trop Med Hyg. 1995 [cited 2023 Jul 20];89(3):262–5. Available from: https://pubmed.ncbi.nlm.nih.gov/7660427/.
Wibawa ID, Muljono DH, Mulyanto, Suryadarma IG, Tsuda F, Takahashi M, et al. Prevalence of antibodies to hepatitis E virus among apparently healthy humans and pigs in Bali, Indonesia: identification of a pig infected with a genotype 4 hepatitis E virus. J Med Virol. 2004;73(1):38–44. Available from: https://pubmed.ncbi.nlm.nih.gov/15042646/.
Goldsmith R, Yarbough PO, Reyes GR, Fry KE, Gabor KA, Kamel M, et al. Enzyme-linked immunosorbent assay for diagnosis of acute sporadic hepatitis E in Egyptian children. Lancet. 1992 [cited 2023 Jul 25];339(8789):328–31. Available from: https://pubmed.ncbi.nlm.nih.gov/1346411/.
Bounlu K, Insisiengmay S, Vanthanouvong K, Saykham, Widjaja S, Iinuma K, et al. Acute jaundice in Vientiane, Lao People’s Democratic Republic. Clin Infect Dis. 1998;27(4):717–21. Available from: https://pubmed.ncbi.nlm.nih.gov/9798023/.
Khounvisith V, Saysouligno S, Souvanlasy B, Billamay S, Mongkhoune S, Vongphachanh B, et al. Hepatitis B virus and other transfusion-transmissible infections in child blood recipients in Lao People’s Democratic Republic: a hospital-based study. Arch Dis Child. 2023;108(1):15–9. Available from: https://pubmed.ncbi.nlm.nih.gov/36344216/.
Khounvisith V, Tritz S, Khenkha L, Phoutana V, Keosengthong A, Pommasichan S, et al. High circulation of hepatitis E virus in pigs and professionals exposed to pigs in Laos. Zoonoses Public Health. 2018;65(8):1020–6. Available from: https://pubmed.ncbi.nlm.nih.gov/30152201/.
Tritz SE, Khounvisith V, Pommasichan S, Ninnasopha K, Keosengthong A, Phoutana V, et al. Evidence of increased hepatitis E virus exposure in Lao villagers with contact to ruminants. Zoonoses Public Health. 2018;65(6):690–701. Available from: https://pubmed.ncbi.nlm.nih.gov/29888475/.
Bisayher S, Barennes H, Nicand E, Buisson Y. Seroprevalence and risk factors of hepatitis E among women of childbearing age in the Xieng Khouang province (Lao People’s Democratic Republic), a cross-sectional survey. Trans R Soc Trop Med Hyg. 2019;113(6):298–304. Available from: https://pubmed.ncbi.nlm.nih.gov/31034060/.
Holt HR, Inthavong P, Khamlome B, Blaszak K, Keokamphe C, Somoulay V, et al. Endemicity of zoonotic diseases in pigs and humans in lowland and upland Lao PDR: identification of socio-cultural risk factors. PLoS Negl Trop Dis. 2016;10(4):e0003913. Available from: https://pubmed.ncbi.nlm.nih.gov/27070428/.
Syhavong B, Rasachack B, Smythe L, Rolain JM, Roque-Afonso AM, Jenjaroen K, et al. The infective causes of hepatitis and jaundice amongst hospitalised patients in Vientiane, Laos. Trans R Soc Trop Med Hyg. 2010;104(7):475–83. Available from: https://pubmed.ncbi.nlm.nih.gov/20378138/.
Chansamouth V, Thammasack S, Phetsouvanh R, Keoluangkot V, Moore CE, Blacksell SD, et al. The aetiologies and impact of fever in pregnant inpatients in Vientiane, Laos. PLoS Negl Trop Dis. 2016;10(4):e0004577.
Wong LP, Tay ST, Chua KH, Goh XT, Alias H, Zheng Z, et al. Serological evidence of Hepatitis E virus (HEV) infection among ruminant farmworkers: a retrospective study from Malaysia. Infect Drug Resist. 2022;15:5533–41. Available from: https://pubmed.ncbi.nlm.nih.gov/36164335/.
Wong LP, Lee HY, Khor CS, Abdul-Jamil J, Alias H, Abu-Amin N, et al. The risk of transfusion-transmitted hepatitis E virus: evidence from seroprevalence screening of blood donations. Indian J Hematol Blood Transfus. 2022;38(1):145–52. Available from: https://pubmed.ncbi.nlm.nih.gov/33879981/.
Wong LP, Alias H, Choy SH, Goh XT, Lee SC, Lim YAL, et al. The study of seroprevalence of hepatitis E virus and an investigation into the lifestyle behaviours of the aborigines in Malaysia. Zoonoses Public Health. 2020;67(3):263–70. Available from: https://pubmed.ncbi.nlm.nih.gov/31927794/.
Ng KP, He J, Saw TL, Lyles CM. A seroprevalence study of viral hepatitis E infection in human immunodeficiency virus type 1 infected subjects in Malaysia. Med J Malaysia. 2000;55(1):58–64. Available from: https://pubmed.ncbi.nlm.nih.gov/11072492/.
Hudu SA, Niazlin MT, Nordin SA, Harmal NS, Tan SS, Omar H, et al. Hepatitis E virus isolated from chronic hepatitis B patients in Malaysia: sequences analysis and genetic diversity suggest zoonotic origin. Alexandria J Med. 2018;54(4):487–94. Available from: https://www.tandfonline.com/doi/pdf/10.1016/j.ajme.2017.07.003.
Anderson DA, Li F, Riddell M, Howard T, Seow HF, Torresi J, et al. ELISA for IgG-class antibody to hepatitis E virus based on a highly conserved, conformational epitope expressed in Escherichia coli. J Virol Methods. 1999 [cited 2023 Jul 25];81(1–2):131–42. Available from: https://pubmed.ncbi.nlm.nih.gov/10488771/.
Seow HF, Mahomed NM, Mak JW, Riddell MA, Li F, Anderson DA. Seroprevalence of antibodies to hepatitis E virus in the normal blood donor population and two aboriginal communities in Malaysia. J Med Virol. 1999;59(2):164–8. Available from: https://pubmed.ncbi.nlm.nih.gov/10459151/.
Saat Z, Sinniah M, Kin TL, Baharuddin R, Krishnasamy M. A four year review of acute viral hepatitis cases in the east coast of Peninsular Malaysia. Southeast Asian J Trop Med Public Health. 1999;30(1):106–9.
Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, et al. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997 [cited 2023 Jul 25];71(10):7207–13. Available from: https://pubmed.ncbi.nlm.nih.gov/9311793/.
Uchida T, Aye TT, Ma X, Iida F, Shikata T, Ichikawa M, et al. An epidemic outbreak of hepatitis E in Yangon of Myanmar: antibody assay and animal transmission of the virus. Acta Pathol Jpn. 1993;43(3):94–8. Available from: https://pubmed.ncbi.nlm.nih.gov/8257479/.
Nakai K, Win KM, Oo SS, Arakawa Y, Abe K. Molecular characteristic-based epidemiology of hepatitis B, C, and E viruses and GB virus C/hepatitis G virus in Myanmar. J Clin Microbiol. 2001;39(4):1536–9. Available from: https://pubmed.ncbi.nlm.nih.gov/11283083/.
Chow WC, Ng HS, Lim GK, Oon CJ. Hepatitis E in Singapore–a seroprevalence study. Singapore Med J. 1996;37(6):579–81. Available from: https://pubmed.ncbi.nlm.nih.gov/9104052/.
Wong CC, Thean SM, Ng Y, Kang JSL, Ng TY, Chau ML, et al. Seroepidemiology and genotyping of hepatitis E virus in Singapore reveal rise in number of cases and similarity of human strains to those detected in pig livers. Zoonoses Public Health. 2019 [cited 2023 Jul 24];66(7):773–82. Available from: https://pubmed.ncbi.nlm.nih.gov/31293095/.
Tan LTC, Tan J, Ang LW, Chan KP, Chiew KT, Cutter J, et al. Epidemiology of acute hepatitis E in Singapore. J Infect. 2013 [cited 2023 Jul 24];66(5):453–9. Available from: https://pubmed.ncbi.nlm.nih.gov/23286967/.
Pourpongporn P, Samransurp K, Rojanasang P, Wiwattanakul S, Srisurapanon S. The prevalence of anti-hepatitis E in occupational risk groups. J Med Assoc Thai. 2009;92:S38–42. Available from: https://pubmed.ncbi.nlm.nih.gov/19705545/.
Siripanyaphinyo U, Boon-Long J, Louisirirotchanakul S, Takeda N, Chanmanee T, Srimee B, et al. Occurrence of hepatitis E virus infection in acute hepatitis in Thailand. J Med Virol. 2014;86(10):1730–5. Available from: https://pubmed.ncbi.nlm.nih.gov/24984976/.
Poovorawan K, Jitmitrapab S, Treeprasertsuk S, Tangkijvanich P, Komolmitr P, Poovorawan Y. Acute hepatitis E in Thailand, 2009–2012. J Gastroenterol Hepatol. 2012;27:233.
Maneerat Y, Wilairatana P, Pongponratn E, Chaisri U, Puthavatana P, Snitbhan R, et al. Etiology of acute non-A, B, C hepatitis in Thai patients: preliminary study. Southeast Asian J Trop Med Public Health. 1996;27(4):844–6. Available from: https://pubmed.ncbi.nlm.nih.gov/9253895/.
Sa-nguanmoo P, Posuwan N, Vichaiwattana P, Wutthiratkowit N, Owatanapanich S, Wasitthankasem R, et al. Swine is a possible source of hepatitis E virus infection by comparative study of hepatitis A and E seroprevalence in Thailand. PLoS One. 2015;10:e0126184. Available from: https://pubmed.ncbi.nlm.nih.gov/25927925/.
Pilakasiri C, Gibbons RV, Jarman RG, Supyapoung S, Myint KSA. Hepatitis antibody profile of Royal Thai Army nursing students. Trop Med Int Health. 2009;14(6):609–11. Available from: https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/j.1365-3156.2009.02264.x?download=true.
Louisirirotchanakul S, Myint KS, Srimee B, Kanoksinsombat C, Khamboonruang C, Kunstadter P, et al. The prevalence of viral hepatitis among the Hmong people of northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33(4):837–44. Available from: https://pubmed.ncbi.nlm.nih.gov/12757235/.
Jupattanasin S, Chainuvati S, Chotiyaputta W, Chanmanee T, Supapueng O, Charoonruangrit U, et al. A nationwide survey of the seroprevalence of hepatitis E virus infections among blood donors in Thailand. Viral Immunol. 2019;32(7):302–7. Available from: https://pubmed.ncbi.nlm.nih.gov/31403386/.
Hinjoy S, Nelson KE, Gibbons RV, Jarman RG, Mongkolsirichaikul D, Smithsuwan P, et al. A cross-sectional study of hepatitis E virus infection in healthy people directly exposed and unexposed to pigs in a rural community in northern Thailand. Zoonoses Public Health. 2013;60(8):555–62. Available from: https://pubmed.ncbi.nlm.nih.gov/23280251/.
Getsuwan S, Pasomsub E, Yutthanakarnwikom P, Tongsook C, Butsriphum N, Tanpowpong P, et al. Seroprevalence of hepatitis E virus after pediatric liver transplantation. J Trop Pediatr. 2023;69(2):fmad011. Available from: https://pubmed.ncbi.nlm.nih.gov/36811578/.
Gonwong S, Chuenchitra T, Khantapura P, Islam D, Sirisopana N, Mason CJ. Pork consumption and seroprevalence of hepatitis E virus, Thailand, 2007–2008. Emerg Infect Dis. 2014;20:1531–4. Available from: https://pubmed.ncbi.nlm.nih.gov/25148245/.
Komolmit P, Oranrap V, Suksawatamnuay S, Thanapirom K, Sriphoosanaphan S, Srisoonthorn N, et al. Clinical significance of post-liver transplant hepatitis E seropositivity in high prevalence area of hepatitis E genotype 3: a prospective study. Sci Rep. 2020;10(1):7352. Available from: https://pubmed.ncbi.nlm.nih.gov/32355268/.
Jutavijittum P, Jiviriyawat Y, Jiviriyawat W, Yousukh A, Hayashi S, Itakura H, et al. Seroprevalence of antibody to hepatitis E virus in voluntary blood donors in Northern Thailand. Trop Med. 2000;42(2):135–9. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-0033638431&partnerID=40&md5=12c324fd502945b8c8dc425274f7cad2.
Boonyai A, Thongput A, Sisaeng T, Phumchan P, Horthongkham N, Kantakamalakul W, et al. Prevalence and clinical correlation of hepatitis E virus antibody in the patients’ serum samples from a tertiary care hospital in Thailand during 2015–2018. Virol J. 2021;18(1):145. Available from: https://pubmed.ncbi.nlm.nih.gov/34247642/.
Huy PX, Chung DT, Linh DT, Hang NT, Rachakonda S, Pallerla SR, et al. Low prevalence of HEV infection and no associated risk of HEV transmission from mother to child among pregnant women in Vietnam. Pathogens. 2021;10(10):1340. Available from: https://pubmed.ncbi.nlm.nih.gov/34684289/.
Hoan NX, Huy PX, Sy BT, Meyer CG, Son TV, Binh MT, et al. High hepatitis E virus (HEV) Positivity among domestic pigs and risk of HEV infection of individuals occupationally exposed to pigs and pork meat in Hanoi, Vietnam. Open Forum Infect Dis. 2019;6(9):ofz306. Available from: https://pubmed.ncbi.nlm.nih.gov/31660396/.
Hoan NX, Tong HV, Hecht N, Sy BT, Marcinek P, Meyer CG, et al. Hepatitis E virus superinfection and clinical progression in hepatitis B patients. EBioMedicine. 2015;2(12):2080–6. Available from: https://pubmed.ncbi.nlm.nih.gov/26844288/.
Hau CH, Hien TT, Tien NT, Khiem HB, Sac PK, Nhung VT, et al. Prevalence of enteric hepatitis A and E viruses in the Mekong River delta region of Vietnam. Am J Trop Med Hyg. 1999;60(2):277–80. Available from: https://pubmed.ncbi.nlm.nih.gov/10072151/.
Corwin AL, Dai TC, Duc DD, Suu PI, Van NT, Ha LD, et al. Acute viral hepatitis in Hanoi, Viet Nam. Trans R Soc Trop Med Hyg. 1996;90(6):647–8. Available from: https://pubmed.ncbi.nlm.nih.gov/9015503/.
Corwin AL, Khiem HB, Clayson ET, Pham KS, Vo TT, Vu TY, et al. A waterborne outbreak of hepatitis E virus transmission in southwestern Vietnam. Am J Trop Med Hyg. 1996;54(6):559–62. Available from: https://pubmed.ncbi.nlm.nih.gov/8686771/.
Berto A, Pham HA, Thao TTN, Vy NHT, Caddy SL, Hiraide R, et al. Hepatitis E in southern Vietnam: seroepidemiology in humans and molecular epidemiology in pigs. Zoonoses Public Health. 2018 [cited 2023 Jul 24];65(1):43–50. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/zph.12364.
Li TC, Zhang J, Shinzawa H, Ishibashi M, Sata M, Mast EE, et al. Empty virus-like particle-based enzyme-linked immunosorbent assay for antibodies to hepatitis E virus. J Med Virol. 2000;62(3):327–33.
Shimizu K, Hamaguchi S, Ngo CC, Li TC, Ando S, Yoshimatsu K, et al. Serological evidence of infection with rodent-borne hepatitis E virus HEV-C1 or antigenically related virus in humans. J Vet Med Sci. 2016 [cited 2023 Jul 24];78(11):1677–81. Available from: https://pubmed.ncbi.nlm.nih.gov/27499185/.
Nghiem XH, Pham XH, Trinh VS, Dao PG, Mai TB, Dam TA, et al. HEV positivity in domesticated pigs and a relative risk of HEV zoonosis among occupationally exposed individuals in Vietnam. J Hepatol. 2018 [cited 2023 Jul 24];68:S186–7. Available from: https://www.researchgate.net/publication/324700980.
Tran HTT, Ushijima H, Quang VX, Phuong N, Li TC, Hayashi S, et al. Prevalence of hepatitis virus types B through E and genotypic distribution of HBV and HCV in Ho Chi Minh City. Vietnam Hepatology Research. 2003 [cited 2023 Jul 24];26(4):275–80. Available from: https://pubmed.ncbi.nlm.nih.gov/12963426/.
South-East Asia | Demographic Changes. Available from: https://www.population-trends-asiapacific.org/data/sea. Accessed May 18 2023.
Sentian J, Payus CM, Herman F, Kong VWY. Climate change scenarios over Southeast Asia. APN Sci Bull. 2022 [cited 2023 Sep 28];12(1):102–22. Available from: https://www.apn-gcr.org/bulletin/?p=1927.
Lee T HJ. Southeast Asia’s growing meat demand and its implications for feedstuffs imports. Amber Waves: The Economics of Food, Farming, Natural Resources, and Rural America. 2019;(03). https://ideas.repec.org/a/ags/uersaw/302703.html. https://www.ers.usda.gov/amber-waves/2019/april/southeast-asia-s-growing-meat-demand-and-its-implications-forfeedstuffs-imports/.
Rossi-Tamisier M, Moal V, Gerolami R, Colson P. Discrepancy between anti-hepatitis E virus immunoglobulin G prevalence assessed by two assays in kidney and liver transplant recipients. J Clin Virol. 2013 [cited 2023 Jul 27];56(1):62–4. Available from: https://pubmed.ncbi.nlm.nih.gov/23089569/.
Wenzel JJ, Preiss J, Schemmerer M, Huber B, Jilg W. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J Infect Dis. 2013 [cited 2023 Jul 27];207(3):497–500. Available from: https://pubmed.ncbi.nlm.nih.gov/23148290/.
Chongsuvivatwong V, Phua KH, Yap MT, Pocock NS, Hashim JH, Chhem R, et al. Health and health-care systems in southeast Asia: diversity and transitions. Lancet. 2011;377(9763):429–37.
Acknowledgements
The authors would like to thank all researchers of the primary research included in this study.
Funding
This work was supported by Project Research Center for Epidemiology and Prevention of Viral Hepatitis and Hepatocellular Carcinoma, Hiroshima University led by Prof. Junko Tanaka (PI).
Author information
Authors and Affiliations
Contributions
UM, TA, and JT conceptualized the study. UM and SO contributed to developing the study design and data acquisition. UM, CC, ZP, AG, SO, and JT analysed and interpreted the data. UM, KK, and AS drafted the manuscript. TA, AS, KK, SO, and JT contributed to the intellectual content of the manuscript. All authors read and approved the final manuscript. JT and TA shared the co-correspondence.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mirzaev, U.K., Ouoba, S., Ko, K. et al. Systematic review and meta-analysis of hepatitis E seroprevalence in Southeast Asia: a comprehensive assessment of epidemiological patterns. BMC Infect Dis 24, 525 (2024). https://doi.org/10.1186/s12879-024-09349-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09349-2