- Research
- Open access
- Published:
A score to predict Pseudomonas aeruginosa infection in older patients with community-acquired pneumonia
BMC Infectious Diseases volume 23, Article number: 700 (2023)
Abstract
Background
In Thailand, the incidence of community-acquired pseudomonal pneumonia among 60- to 65-year-olds ranges from 10.90% to 15.51%, with a mortality rate of up to 19.00%. Antipseudomonal agents should be selected as an empirical treatment for elderly patients at high risk for developing this infection. The purpose of this study was to identify risk factors and develop a risk predictor for Pseudomonas aeruginosa infection in older adults with community-acquired pneumonia (CAP).
Methods
A retrospective data collection from an electronic database involved the elderly hospitalized patients with P. aeruginosa- and non-P. aeruginosa-causing CAP, admitted between January 1, 2016, and June 30, 2021. Risk factors for P. aeruginosa infection were analysed using logistic regression, and the instrument was developed by scoring each risk factor based on the beta coefficient and evaluating discrimination and calibration using the area under the receiver operating characteristic curve (AuROC) and observed versus predicted probability (E/O) ratio.
Results
The inclusion criteria were met by 81 and 104 elderly patients diagnosed with CAP caused by P. aeruginosa and non-P. aeruginosa, respectively. Nasogastric (NG) tube feeding (odd ratios; OR = 40.68), bronchiectasis (B) (OR = 4.13), immunocompromised condition (I) (OR = 3.76), and other chronic respiratory illnesses (r) such as atelectasis, pulmonary fibrosis, and lung bleb (OR = 2.61) were the specific risk factors for infection with P. aeruginosa. The “60-B-r-I-NG” risk score was named after the 4 abbreviated risk variables and found to have good predicative capability (AuROC = 0.77) and accuracy comparable to or near true P. aeruginosa infection (E/O = 1). People who scored at least two should receive empirically antipseudomonal medication.
Conclusions
NG tube feeding before admission, bronchiectasis, immunocompromisation, atelectasis, pulmonary fibrosis and lung bleb were risk factors for pseudomonal CAP in the elderly. The 60-B-r-I-NG was developed for predicting P. aeruginosa infection with a high degree of accuracy, equal to or comparable to the existing P. aeruginosa infection. Antipseudomonal agents may be started in patients who are at least 60 years old and have a score of at least 2 in order to lower mortality and promote the appropriate use of these medications.
Background
Community-acquired pneumonia (CAP) is lung infection that started outside the hospital or within 48 h of hospital admission [1]. It is a prevalent infectious disease affecting the elderly, with more than 30–40% of cases hospitalized [2]. Klebsiella pneumoniae (20.4%), P. aeruginosa (15.5%), methicillin-susceptible Staphylococcus aureus (8.9%), Acinetobacter baumannii (7.3%), Haemophilus parainfluenzae (6.7%), H. influenzae (5.9%), and Streptococcus pneumoniae (5.9%) were identified as the causative agents of CAP in Thai patients aged 60 or older [3]. In addition, 60.80% of P. aeruginosa lung infections were determined to be severe, which was twice as high as for other pathogens. Compared to other pathogens’ mortality rate of 5.5%, the 30-day mortality rate associated with P. aeruginosa infections was statistically substantially higher (18.20%), and the risk of 30-day mortality was 2.40 times higher than that of others [4].
A review of the literature revealed substantial numbers of risk factors for P. aeruginosa infection in elderly patients including previous P. aeruginosa infection or colonization within the past year (OR 16.10–19.20), enteral tube feeding (OR 13.90), tracheostomy (OR 6.50–6.95), bronchiectasis (OR 2.90–6.10), male (OR 3.71), lung abscess or empyema (OR 3.4), chronic obstructive pulmonary disease (COPD) (OR 1.8–2.89), indwelling catheters, including central venous and bladder catheters (OR 2.49), other chronic respiratory diseases (OR 2.30), and use of inhaled corticosteroids (OR 1.76) [4,5,6,7,8,9].
To help reduce mortality from CAP caused by P. aeruginosa infection, infection risk prediction tools are therefore essential. Currently, there is no specific predictive tool for assessing the infection risk of P. aeruginosa in elderly CAP patients.
This study was conducted to identify risk factors and develop a predictive tool for the risk of CAP caused by P. aeruginosa in the elderly in order to develop a guideline for the use of antipseudomonal agents as empiric treatment.
Methods
Study design and setting
An analysis of older patients with CAP admitted to a single general hospital with 1,000 beds between January 1, 2016, and June 30, 2021, was done retrospectively. CAP was identified according to the International Classification of Diseases, 10th Revision (ICD-10).
Inclusion and exclusion criteria
Eligible patients were at least 60 years old, had pneumonia with new pulmonary infiltration on chest radiograph and respiratory symptoms including fever, coughing, or pleuritic chest pain, had not been hospitalized within the previous 14 days, and had a pathogen identified from sputum culture. Patients who had pneumonia together with other organ infections (apart from bloodstream infections) or who lacked information needed to determine risk factors were excluded from the study.
Definitions
Elderly were defined as patients were at least 60 years old according to Thai criteria.
Chronic respiratory diseases were categorized into three groups: COPD, bronchiectasis, and others.
Immunocompromised status was defined as: receiving prednisone greater than 15 mg/day for longer than 2 weeks or stopped within the past 2 weeks; having a neutrophil count of 500/mm3 or less; being on immunosuppressive therapy; presenting with active solid organ/haematologic malignancies or receiving chemotherapy within the past 6 months; having an human immunodeficiency virus (HIV) infection with a CD4+ lymphocyte count of less than 200/µL; or having received a solid organ or hematopoietic stem cell transplant within the previous year.
Severe CAP was defined according to the criteria outlined by the Infectious Diseases Society of America (IDSA) and The American Thoracic Society (ATS) as follows: having at least one major criterion (invasive mechanical ventilation; septic shock requiring vasopressors) or having three of the minor criteria (a respiratory rate of at least 30 breaths/min; a PaO2/FiO2 ratio of no more than 250; multilobar infiltrates; confusion/disorientation; uremia with a BUN level of at least 20 mg/dL; leukopenia with a WBC count less than 4,000 cells/mm3; thrombocytopenia with a platelet count less than 100,000 cells/mm3; hypothermia with a core temperature less than 36°C; and hypotension requiring aggressive fluid resuscitation).
Malnutrition was defined as having a BMI of less than 18 kg/m2.
Data collection
Baseline patient characteristics, microbiological findings, and factors for risk factors for P. aeruginosa infection were gathered from the electronic Hospital Information System database, which is used in hospitals across Thailand. The data was collected using a spreadsheet by a single researcher.
Sputum collection methods
Standard microbiological methods were used to analyze the pathogens in sputum. Sputum from the patient’s respiratory tract was obtained through coughing or suction, and ward nurses who had received training in the practical guidelines for collecting specimens for the diagnosis of respiratory tract infections collected it into sterile containers and immediately transported it to the microbiology laboratory. Good-quality sputum samples (with more than 25 polymorphonuclear cells and fewer than 10 epithelial cells per low-power field at a total magnification of × 100) were cultured sequentially on chocolate agar, sheep blood agar, and MacConkey agar. Pathogens were identified using biochemical tests or an automated system.
Statistical analysis
All the data were analysed using version 14.1 of STATA (StataCorp LLC, College Station, Texas, USA). Depending on the data type, patients’ characteristics were presented as frequency and percentage, mean ± SD or median, and interval range (IQR). Baseline characteristics between the patients with pseudomonal and non-pseudomonal CAP were compared using the chi-squared or Fisher’s exact test. Our research employed a two-sided alpha error of 0.05, so a p-value less than 0.05 is statistically significant.
During model development, relevant independent variables (p-value < 0.20) were incorporated using bivariate selection. As P. aeruginosa infection was the anticipated outcome, multivariable logistic regression was employed, and the OR and 95% confidence interval (95%CI) were reported. We conducted a complete-case analysis on every result.
Constructing a risk prediction model
Assignment of weights to each risk factor
According to Sullivan et al. [10], the beta coefficients for each risk factor in the multivariable logistic regression analysis were calculated to provide a point-based system. The constant (C), which matched the beta coefficient of the risk factor with the lowest value, was then established. Then, in order to assign scores to each risk factor, the beta coefficient of each additional risk factor was divided by the magnitude of C.
Model performance
The area under the receiver operating characteristic curve (AuROC), a comparison of true positive rate (TPR) indicating the presence of P. aeruginosa and the false positive rate (FPR) indicating the absence of P. aeruginosa as the criterion changes, was used to evaluate discrimination, where an AuROC of less than 0.500, 0.500–0.699, 0.700–0.799, 0.800–0.899, and at least 0.900 indicates no, low, good, very good, and excellent predictive power, respectively.
E/O represents the ratio between the expected and observed number of events, with E/O less than 1.00, equal to 1.00, and greater than 1.00 indicating that the model predicts that the incidence of P. aeruginosa infection is less than, equal to or nearly equal to, and greater than the actual incidence, respectively.
-
Likelihood ratio for a positive result (LR+)
$$LR+ = \frac{Sensitivity}{1-Specitivity}$$ -
Likelihood ratio for a negative result (LR-)
$$LR- = \frac{1-Sensitivity}{Specitivity}$$ -
Accuracy (proportional of correctly classified subjects, CCR)
$$CCR=\left(\frac{TP+TN}{TP+FP+TN+FN}\right)\times 100$$ -
TP, true positive; FP, false positive; FN, false negative; TN, true negative
Internal validation
The bootstrap validation method was used for an internal validation [11]. We ran 500 cycles of bootstrapping and calculated the average optimism as the difference between the bootstrap and test performance in terms of AuROC, calibration-in-the-large, and calibration slope. To retrieve the optimism-corrected model, we utilized the uniform shrinkage method and carried out the following three steps: first, a constant term was subtracted from the linear predictor. Then, all coefficient values were multiplied by the optimism-adjusted AuROC derived from the internal validation. The new constant term was finally recalculated. In addition, we created a calibration plot based on the optimism-adjusted model.
Refitting method
The Cslope derived from internal validation was utilized to calculate the tool’s intercept. AuROC and E/O were then used to evaluate the predictive power of the tool and the accuracy of P. aeruginosa infection, respectively.
Optimal cut-off point selection
The issue was the selection of a cut-off point with adequate sensitivity and specificity for predicting P. aeruginosa infection risk using the Youden index (J) method. An appropriate threshold for the instrument used to predict the risk of P. aeruginosa infection [J = sensitivity − (1 − specificity)] or consider the probability of P. aeruginosa infection for each tool score.
Results
Among 185 senior CAP patients, 81 (43.78%) had P. aeruginosa identified from sputum cultures, whereas 104 of them had infection from other pathogens, including Klebsiella pneumoniae (56.73%), Streptococcus pneumoniae (19.23%), Haemophilus influenzae (8.65%), Staphylococcus aureus (5.77%), Escherichia coli (5.77%), and others (3.85%). Significantly more frequently than in the latter group, the P. aeruginosa group displayed the following characteristics: bronchiectasis, other chronic respiratory diseases, including atelectasis, pulmonary fibrosis, and lung bleb, immunosuppressed condition (those who have receiving prednisone greater than 15 mg/day for longer than 2 weeks or stopped within the past 2 weeks, having neutrophil count less than 500/mm3, being on immunosuppressive therapy, presenting with active solid organ/haematologic malignancies or receiving chemotherapy within the past 6 months, and having human immunodeficiency virus (HIV) infection with a CD4+ lymphocyte count of less than 200/µL were 5, 2, 24, and 2 cases, respectively), tracheostomy or NG tube feeding prior to hospitalization, use of a ventilator or intravenous antimicrobial therapy, or hospitalization within the previous 3 months (Table 1). For this study, the impact of receiving oral corticosteroids on P. aeruginosa infection was not investigated due to the limited number of patients, as previously mentioned.
As shown in Table 1, patients with P. aeruginosa infections exhibited severe symptoms in two-thirds of cases, compared to 30% of patients with non-P. aeruginosa infection. The proportion of men, the prevalence of COPD, the use of inhaled corticosteroids or proton pump inhibitors before hospitalization, malnutrition, bedridden patients, chronic heart failure, cerebrovascular disease, chronic neurological disorders, chronic kidney disease with GFR less than 35 mL/min/1.73 m2, type 2 diabetes mellitus, alcohol use, or smoking were not statistically different between the two groups.
Factors associated with P. aeruginosa infection in the elderly with CAP
Tracheostomy before hospitalization, use of a ventilator in the last 3 months, receiving intravenous antimicrobials within the past 3 months, history of hospitalization within the past 3 months, malnutrition, taking inhaled corticosteroids before hospitalization, COPD, and alcohol consumption were the major risk factors from univariate logistic regression analysis that were not significant in multivariate logistic regression analysis.
The following elements were shown to still be statistically significant after multivariate logistic regression analysis: NG tube feeding prior to admission, bronchiectasis, immunosuppressed condition, and other chronic respiratory diseases, which include atelectasis, pulmonary fibrosis, and lung bleb. These factors increased the risk of P. aeruginosa infection by 40.98 (OR 40.98; 95%CI 3.85–429.33), 4.13 (OR 4.13; 95%CI 1.28–13.34), 3.67 (OR 3.76; 95%CI 1.23–11.51), and 2.61 (OR 2.61; 95%CI 1.05–6.44) times, respectively, when compared to individuals without these characteristics (Table 2).
Constructing a risk prediction model
Assignment of weights to each risk factor
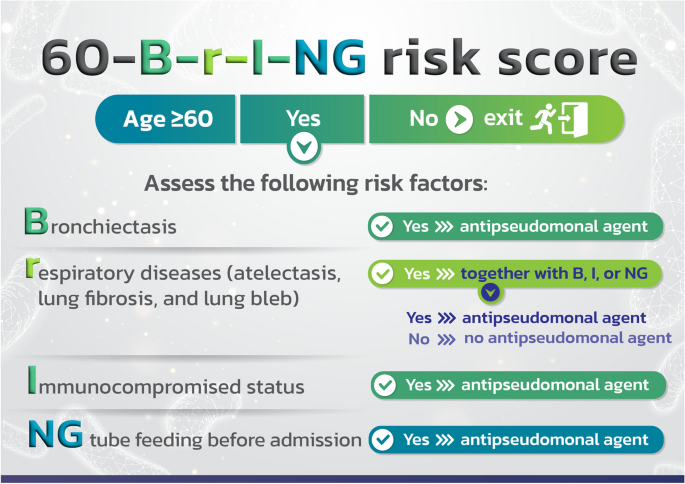
The beta coefficient for each risk factor was determined. As can be seen in Table 3, the beta coefficients for NG tube feeding before admission (NG), bronchiectasis (B), and immunosuppressed status (I) were divided by the lowest value for other chronic respiratory disease (r) to get scores of 4, 2, and 2 correspondingly. To make the tool more recognizable and to underline that it is used to predict the likelihood of P. aeruginosa infection in CAP patients 60 years of age and above, we gave it the name 60-B-r-I-NG risk score (Fig. 1).
Model performance
With an AuROC of 0.77, the 60-B-r-I-NG risk score had a good ability to predict the presence of P. aeruginosa infection (Fig. 2a).
Based on a comparison of the predicted risk of P. aeruginosa infection with the observed risk, Fig. 2b demonstrates that the calibration of the 60-B-r-I-NG risk score was at or near the actual incidence of P. aeruginosa infection (E/O = 1).
Internal validation and refitting method
Using bootstrap validation, the 60-B-r-I-NG risk score for each randomization was compared to the presence of P. aeruginosa infection after 500 randomizations of patient data, obtaining a Cslope of 0.96 (not shown), which showed that the proportion of P. aeruginosa group and non-P. aeruginosa group was similar to that in the original patient group. However, recalibration was carried out to better extrapolate the risk score to other populations by recalculating the intercept and slope based on our data. The Cslope result of 1.038, with an AuROC and E/O remaining the same (0.77 and 1, respectively), showing that this instrument can be applied to other populations (Fig. 3).
Optimal cut-off point selection
Using the 60-B-r-I-NG risk score to predict P. aeruginosa infection risk, the optimal score was determined by calculating the sensitivity and specificity of each score using the Youden index. The highest and most comparable Youden index values for the 60-B-r-I-NG risk score were 0.47 and 0.46, with sensitivity and specificity values of 67.90% and 78.85%, and 59.26% and 86.54%, respectively (Table 4). Therefore, the cut-off points 1 or 2 was could be used to predict the risk of P. aeruginosa infection. However, taking into account that when the cut-off score was 2 points, the likelihood of P. aeruginosa infection (66.84 percent) was higher (Fig. 4). The authors came to the conclusion that a 60-B-r-I-NG risk score of at least 2 suggests a risk for P. aeruginosa infection in elderly patients with CAP.
Discussion
Even while P. aeruginosa only causes severe CAP in only 1.8% to 8.3% of patients, it contributes to a high mortality rate of 50% to 100% [4]. Therefore, empiric therapy for patients with suspected P. aeruginosa infection is necessary to reduce mortality.
According to country-specific treatment guidelines, the risk factors for P. aeruginosa pulmonary infection vary. These include severe underlying lung disease, recurrent bronchiectasis, use of antibiotics within the previous 3 months, recent hospitalization, airway P. aeruginosa colonization, history of antibiotic therapy for 2 or more days within the previous 90 days, current tube feeding, and alcohol consumption [12,13,14,15,16,17,18].
A history of respiratory isolation of P. aeruginosa during the previous year and hospitalization with taking parenteral antibiotics within the preceding 90 days are both identified as risk factors for P. aeruginosa infection in the most recent 2019 treatment guidelines from the IDSA and ATS [1]. However, they stress that the most crucial ones are locally confirmed risk factors for P. aeruginosa infections because prior research from various population and geographic studies has produced inconsistent results about the associations between risk factors and P. aeruginosa infections.
The following risk factors were of particular interest: infection with or colonization by P. aeruginosa within the past year (OR 16.10; 95%CI 9.48–27.35) [6], enteral tube feeding (OR 13.87; 95%CI 3.39–56.65) [8], tracheostomy (OR 6.50; 95%CI 2.61–16.19) [6], hospitalization for more than 2 days within the past 30 days but not within the past 7 days (OR 3.8; 95%CI 1.8–8.3) [19], male (OR 3.71; 95%CI 1.65–8.35) [4], and immunodeficiency (OR 1.39; 95%CI 1.22–1.58) [20]. Despite definitional differences, the majority of studies have found that bronchial or pulmonary diseases, including asthma, uncomplicated chronic bronchitis, COPD, bronchiectasis, and interstitial lung disease, are risk factors for P. aeruginosa infection (OR 1.25–5.8; p < 0.05) [4, 6, 8, 19]. In addition, severe COPD or pneumonia necessitating mechanical ventilation or vasopressors, and having a pneumonia severity index (PSI) risk class of IV–V, are considered high risk (OR 1.85–3.95) [4, 6, 20]. Interestingly, recent exposure to inhaled corticosteroids within the past 90 days (OR 1.40; 95%CI 1.23–1.61), receiving Gram-positive coverage therapy within the past 90 days (OR 1.37; 95%CI 1.01–1.87), prior hospitalization within the past 90 days (OR 1.36; 95%CI 1.21–1.54), and use of beta-lactams within the past 90 days (OR 1.31; 95%CI 1.14–1.51) are weakly associated with P. aeruginosa infection [19]. One study also discovered that having diabetes (OR 0.82; 95%CI 0.70–0.95) and being older than 84 years old (OR 0.64; 95%CI 0.52–0.78) decreased the likelihood of contracting this pathogen [20].
One benefit of this study was the tool’s ability to precisely examine risk factors for P. aeruginosa infection while various predictive scores were developed to evaluate the risk of infection from not only P. aeruginosa but also methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, Stenotrophomonas maltophilia, enterococci, and Acinetobacter baumannii, in patients with community-onset, healthcare-associated pneumonia [21,22,23,24,25,26,27,28,29,30]. Although not all of the studies listed in Table 5 provided evidence that our identified risk factors, such as NG tube feeding, bronchiectasis, or immunocompromised, were risk factors for P. aeruginosa infection, we believed that our instrument developed based on locally validated risk factors that have been shown to influence P. aeruginosa infection in patients over 60 years of age diagnosed with CAP. Another advantage was that only our study found a link between pseudomonal pneumonia and lung bleb, atelectasis, and pulmonary fibrosis.
This study identified NG tube feeding prior to admission as the strongest predictor of P. aeruginosa infection. The organism can be found in the environment, particularly in water. After entering the body via the respiratory tract, biofilms are easily formed on the inner surface of tubes [31]. In one study, P. aeruginosa was cultured from the tongue dorsal swabs of 34% of elderly patients who wore NG tubes for at least 2 weeks, whereas no such bacteria were found in the group without NG tubes (50 cases; p < 0.001). Scanning electron micrography analysis of samples from the oropharyngeal section of the NG tube revealed that the biofilm was produced by the same strain of P. aeruginosa found in the oropharynx of P. aeruginosa-infected patients [32]. NG tube feeding being a significant risk factor for P. aeruginosa is thus not surprising.
Due to primary antibody deficiencies, bronchiectasis is a risk factor for the development of CAP [33], and P. aeruginosa is the leading cause of this disease. When it binds to the airway epithelium with its flagella and pili [34], P. aeruginosa secretes various virulence factors that promote cell adhesion and tissue invasion, inhibit mucociliary function, and dysregulate host immunity, leading to airway inflammation and tissue damage. With bronchiectasis, infection and inflammation occur simultaneously in the trachea. This creates favourable conditions for the colonization of pathogens, particularly P. aeruginosa [35], through biofilm formation [34], with the severity of inflammation and the amount of colonization correlated with the severity and frequency of bronchiectasis exacerbations [35].
Immunocompromised patients, particularly those with neutrophil counts less than 500/mm3, haematologic malignancies, transplant recipients, and HIV infection, are at increased risk for P. aeruginosa infection of the pulmonary and circulatory system [34, 36, 37] due to loss of mucosal barriers, mucositis from chemotherapy, and selective pressure from broad-spectrum antimicrobial therapy [36].
The majority of the research participants with P. aeruginosa infection experienced atelectasis (73.08%). This anomaly encourages the production of biofilms, making it a risk factor for P. aeruginosa infection.
COPD prevalence did not differ between the P. aeruginosa and non-P. aeruginosa groups, ruling out a link between COPD and P. aeruginosa infection in this study. P. aeruginosa is typically detected in the sputum of 4% to 15% of COPD patients without a pulmonary infection. In the lungs of COPD patients, there are two types of colonization: short-term colonization followed by eradication and long-term persistence [38]. COPD patients with pulmonary P. aeruginosa infection are associated with a higher incidence of acute COPD exacerbations (AECOPD). It also causes chronic infections in the aforementioned patients. Studies indicated that P. aeruginosa infection can persist in the lungs of COPD patients for up to a year. Compared to bloodstream isolates from non-AECOPD patients, respiratory samples from AECOPD patients tend to have lower cytotoxicity and motility but produce more biofilm in chronic infections [39].
This study was unable to establish a correlation between a previous infection or colonization with P. aeruginosa and the risk of developing a P. aeruginosa infection. As this factor was present in only 9 of 185 patients (4.86%) and all cases were infected with P. aeruginosa, it was not possible to calculate the OR for comparing the presence of these risk factors for infection with P. aeruginosa or other pathogens.
This study has limitations due to the relatively small number of participants, primarily because a high percentage (44.90%) of patients diagnosed with pneumonia in our setting had no bacterial growth in sputum culture. This result was in line with those of a retrospective cohort research carried out at a university hospital in Thailand, which revealed that no bacteria were discovered in sputum cultures 55.15 percent of the time [40]. Furthermore, it was observed that some patients who were initially included in the study were later excluded due to concomitant infections in other organs or insufficient data for identifying risk factors, accounting for 22.00% and 4.00%, respectively.
We allocated scores of 4, 2, 2, and 1 for NG tube feeding prior to admission, bronchiectasis, immunosuppressed state, and other chronic respiratory disease, respectively, nevertheless, the cut-off score for the risk of P. aeruginosa infection was only 2 points. With the exception of having atelectasis, pulmonary fibrosis, and lung bleb, patients are at risk for contracting P. aeruginosa infection even if they only have one risk factor. Therefore, we changed the risk score to the 60-B-r-I-NG checklist (as shown in Fig. 5). CAP cases with NG tube feeding, bronchiectasis, and immunocompromised status should receive empirically antipseudomonal agent based on local susceptibility. If they merely have atelectasis, pulmonary fibrosis, or lung bleb, they do not require antipseudomonal agent.
Conclusions
According to multivariate logistic regression, NG tube feeding prior to admission, bronchiectasis, immunocompromised status, atelectasis, pulmonary fibrosis, or lung bleb were risk factors associated with P. aeruginosa infection in older adults with CAP. The risk score 60-B-r-I-NG was created, and it was discovered to have a high level of prediction power and accuracy, on par with a true P. aeruginosa infection. Elderly CAP patients with a risk score of 2 points or above should have empirical antipseudomonal agent treatment, according to the assessment of the 60-B-r-I-NG risk score.
Availability of data and materials
All the data supporting our findings are contained within the manuscript.
Abbreviations
- AECOPD:
-
Acute chronic obstructive pulmonary disease exacerbations
- AUROC:
-
Area under the receiver operating curve
- B-r-I-NG:
-
Bronchiectasis, respiratory disease, immunocompromised patients, and nasogastric tube feeding
- CAP:
-
Community-acquired pneumonia
- COPD:
-
Chronic obstructive pulmonary disease
- E/O:
-
Observed versus predicted probability
- HIV:
-
Human immunodeficiency virus
- NG:
-
Nasogastric
- P. aeruginosa :
-
Pseudomonas aeruginosa
References
Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67.
Henig O, Kaye KS. Bacterial pneumonia in older adults. Infect Dis Clin North Am. 2017;31(4):689–713.
Osman M, Manosuthi W, Kaewkungwal J, Silachamroon U, Mansanguan C, Kamolratanakul S, et al. Etiology, clinical course, and outcomes of pneumonia in the elderly: a retrospective and prospective cohort study in Thailand. Am J Trop Med Hyg. 2021;104(6):2009–16.
Cillóniz C, Gabarrús A, Ferrer M, Puig de la Bellacasa J, Rinaudo M, Mensa J, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415–25.
Sahuquillo-Arce JM, Menéndez R, Méndez R, Amara-Elori I, Zalacain R, Capelastegui A, et al. Age-related risk factors for bacterial aetiology in community-acquired pneumonia. Respirology. 2016;21(8):1472–9.
Restrepo MI, Babu BL, Reyes LF, Chalmers JD, Soni NJ, Sibila O, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2):1701190.
Lewis PO. Risk factor evaluation for methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in community-acquired pneumonia. Ann Pharmacother. 2021;55(1):36–43.
von Baum H, Welte T, Marre R, Suttorp N, Ewig S. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35(3):598–605.
Di Pasquale MF, Sotgiu G, Gramegna A, Radovanovic D, Terraneo S, Reyes LF, et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis. 2019;68(9):1482–93.
Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631–60.
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73.
Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Summary of Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Disease Society and the Canadian Thoracic Society. Can J Infect Dis. 2000;11(5):237–48.
Menéndez R, Torres A, Aspa J, Capelastegui A, Prat C, Rodríguez de Castro F. Community acquired pneumonia. New guidelines of the Spanish Society of Chest Diseases and Thoracic Surgery (SEPAR). Arch Bronconeumol. 2010;46(10):543–58.
Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect. 2011;17 Suppl 6(Suppl 6):E1–59.
Spindler C, Strålin K, Eriksson L, Hjerdt-Goscinski G, Holmberg H, Lidman C, et al. Swedish guidelines on the management of community-acquired pneumonia in immunocompetent adults--Swedish Society of Infectious Diseases 2012. Scand J Infect Dis. 2012;44(12):885–902.
Cao B, Huang Y, She DY, Cheng QJ, Fan H, Tian XL, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Respir J. 2018;12(4):1320–60.
Mikasa K, Aoki N, Aoki Y, Abe S, Iwata S, Ouchi K, et al. JAID/JSC guidelines for the treatment of respiratory infectious diseases: the Japanese Association for Infectious Diseases/Japanese Society of Chemotherapy - the JAID/JSC guide to clinical management of infectious disease/guideline-preparing committee respiratory infectious disease WG. J Infect Chemother. 2016;22(7 Suppl):S1–s65.
Lee MS, Oh JY, Kang CI, Kim ES, Park S, Rhee CK, et al. Guideline for antibiotic use in adults with community-acquired pneumonia. Infect Chemother. 2018;50(2):160–98.
Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, et al. Community-acquired pneumonia due to gram-negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162(16):1849–58.
Metersky ML, Frei CR, Mortensen EM. Predictors of Pseudomonas and methicillin-resistant Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology. 2016;21(1):157–63.
Aliberti S, Di Pasquale M, Zanaboni AM, Cosentini R, Brambilla AM, Seghezzi S, et al. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis. 2012;54(4):470–8.
Shorr AF, Zilberberg MD, Reichley R, Kan J, Hoban A, Hoffman J, et al. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis. 2012;54(2):193–8.
Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985–95.
Falcone M, Russo A, Giannella M, Cangemi R, Scarpellini MG, Bertazzoni G, et al. Individualizing risk of multidrug-resistant pathogens in community-onset pneumonia. PLoS One. 2015;10(4):e0119528.
El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39(4):474–80.
Schreiber MP, Chan CM, Shorr AF. Resistant pathogens in nonnosocomial pneumonia and respiratory failure: is it time to refine the definition of health-care-associated pneumonia? Chest. 2010;137(6):1283–8.
Park SC, Kang YA, Park BH, Kim EY, Park MS, Kim YS, et al. Poor prediction of potentially drug-resistant pathogens using current criteria of health care-associated pneumonia. Respir Med. 2012;106(9):1311–9.
Prina E, Ranzani OT, Polverino E, Cillóniz C, Ferrer M, Fernandez L, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153–60.
Ma HM, Ip M, Woo J, Hui DS. Development and validation of a clinical risk score for predicting drug-resistant bacterial pneumonia in older Chinese patients. Respirology. 2014;19(4):549–55.
Song JU, Park HK, Kang HK, Lee J. Proposed risk factors for infection with multidrug-resistant pathogens in hemodialysis patients hospitalized with pneumonia. BMC Infect Dis. 2017;17(1):681.
Tuon FF, Dantas LR, Suss PH, TascaRibeiro VS. Pathogenesis of the Pseudomonas aeruginosa biofilm: a review. Pathogens. 2022;11(3):300.
Leibovitz A, Dan M, Zinger J, Carmeli Y, Habot B, Segal R. Pseudomonas aeruginosa and the oropharyngeal ecosystem of tube-fed patients. Emerg Infect Dis. 2003;9(8):956–9.
Quinti I, Soresina A, Guerra A, Rondelli R, Spadaro G, Agostini C, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol. 2011;31(3):315–22.
Reynolds D, Kollef M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: an update. Drugs. 2021;81(18):2117–31.
Vidaillac C, Chotirmall SH. Pseudomonas aeruginosa in bronchiectasis: infection, inflammation, and therapies. Expert Rev Respir Med. 2021;15(5):649–62.
Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171(11):1209–23.
Tofas P, Samarkos M, Piperaki ET, Kosmidis C, Triantafyllopoulou ID, Kotsopoulou M, et al. Pseudomonas aeruginosa bacteraemia in patients with hematologic malignancies: risk factors, treatment and outcome. Diagn Microbiol Infect Dis. 2017;88(4):335–41.
Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(8):853–60.
Martínez-Solano L, Macia MD, Fajardo A, Oliver A, Martinez JL. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(12):1526–33.
Poovieng J, Sakboonyarat B, Nasomsong W. Bacterial etiology and mortality rate in community-acquired pneumonia, healthcare-associated pneumonia and hospital-acquired pneumonia in Thai university hospital. Sci Rep. 2022;12(1):9004.
Acknowledgements
We wish to thank the officials of Buddhachinaraj Hospital for support data. We would like to thank Dr Nat Na-ek and Dr Phaweesa Chawalitpongpun (School of Pharmaceutical Sciences at University of Phayao) for assistance with statistical analysis.
Funding
None
Author information
Authors and Affiliations
Contributions
KW and PS participated in the conception and design of the study, analyzed the data, data interpretation and drafting and revising of the manuscript. JD participated in data management and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research project was approved by the Human Research Ethics Committee of Naresuan University (COA No. 480/2021) and Buddhachinaraj Hospital (COA No. 112/2021) on November 22 and December 20, 2021, respectively. All methods were carried out in accordance with relevant guidelines and regulations in the Declaration of Helsinki. Informed consent was waived because of the retrospective nature of the study by Naresuan University Institutional Review Board (NU-IRB) and Buddhachinaraj Phitsanulok Hospital Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wijit, K., Sonthisombat, P. & Diewsurin, J. A score to predict Pseudomonas aeruginosa infection in older patients with community-acquired pneumonia. BMC Infect Dis 23, 700 (2023). https://doi.org/10.1186/s12879-023-08688-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08688-w